 “Autism Spectrum Disorders comprise conditions that may affect cognitive development, motor skills, social interaction, communication, and behavior. This set of functional deficits often results in lack of independence for the diagnosed individuals, and severe distress for patients, families, and caregivers.
“Autism Spectrum Disorders comprise conditions that may affect cognitive development, motor skills, social interaction, communication, and behavior. This set of functional deficits often results in lack of independence for the diagnosed individuals, and severe distress for patients, families, and caregivers.
There is a mounting body of evidence indicating the effectiveness of pure cannabidiol (CBD) and CBD-enriched Cannabis sativa extract (CE) for the treatment of autistic symptoms in refractory epilepsy patients. There is also increasing data support for the hypothesis that non-epileptic autism shares underlying etiological mechanisms with epilepsy.
Here we report an observational study with a cohort of 18 autistic patients undergoing treatment with compassionate use of standardized CBD-enriched CE (with a CBD to THC ratio of 75/1).
Among the 15 patients who adhered to the treatment (10 non-epileptic and five epileptic) only one patient showed lack of improvement in autistic symptoms. Due to adverse effects, three patients discontinued CE use before 1 month.
After 6-9 months of treatment, most patients, including epileptic and non-epileptic, showed some level of improvement in more than one of the eight symptom categories evaluated: Attention Deficit/Hyperactivity Disorder; Behavioral Disorders; Motor Deficits; Autonomy Deficits; Communication and Social Interaction Deficits; Cognitive Deficits; Sleep Disorders and Seizures, with very infrequent and mild adverse effects.
The strongest improvements were reported for Seizures, Attention Deficit/Hyperactivity Disorder, Sleep Disorders, and Communication and Social Interaction Deficits. This was especially true for the 10 non-epileptic patients, nine of which presented improvement equal to or above 30% in at least one of the eight categories, six presented improvement of 30% or more in at least two categories and four presented improvement equal to or above 30% in at least four symptom categories.
Ten out of the 15 patients were using other medicines, and nine of these were able to keep the improvements even after reducing or withdrawing other medications.
The results reported here are very promising and indicate that CBD-enriched CE may ameliorate multiple ASD symptoms even in non-epileptic patients, with substantial increase in life quality for both ASD patients and caretakers.”
https://www.ncbi.nlm.nih.gov/pubmed/31736860
“The findings presented here, taken together, support the notion that many autism symptoms are associated to neuronal hyperexcitability, and indicate that CBD-enriched CE yields positive effects in multiple autistic symptoms, without causing the typical side effects found in medicated ASD patients. Most patients in this study had improved symptoms even after supervised weaning of other neuropsychiatric drugs.”
https://www.frontiersin.org/articles/10.3389/fneur.2019.01145/full
 “Cannabinoids have been shown to exert analgesic and anti-inflammatory effects, and the effects of cannabinoids are mediated primarily by cannabinoid receptors 1 and 2 (CB1 and CB2).
“Cannabinoids have been shown to exert analgesic and anti-inflammatory effects, and the effects of cannabinoids are mediated primarily by cannabinoid receptors 1 and 2 (CB1 and CB2).
 “Delayed neurologic sequelae (DNS) are among the most serious complications of carbon monoxide (CO) poisoning caused partly by elevated neuroinflammation.
“Delayed neurologic sequelae (DNS) are among the most serious complications of carbon monoxide (CO) poisoning caused partly by elevated neuroinflammation.
 “Autism Spectrum Disorders comprise conditions that may affect cognitive development, motor skills, social interaction, communication, and behavior. This set of functional deficits often results in lack of independence for the diagnosed individuals, and severe distress for patients, families, and caregivers.
“Autism Spectrum Disorders comprise conditions that may affect cognitive development, motor skills, social interaction, communication, and behavior. This set of functional deficits often results in lack of independence for the diagnosed individuals, and severe distress for patients, families, and caregivers. “This paper aimed to systematically examine the efficacy and adverse event (AE) profile of
“This paper aimed to systematically examine the efficacy and adverse event (AE) profile of  “Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder with no known cure and with an average life expectancy of 3-5 years post diagnosis.
“Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder with no known cure and with an average life expectancy of 3-5 years post diagnosis. “Cannabis sativa L. is one of the most-studied species for its phytochemistry due to the abundance of secondary metabolites, including
“Cannabis sativa L. is one of the most-studied species for its phytochemistry due to the abundance of secondary metabolites, including 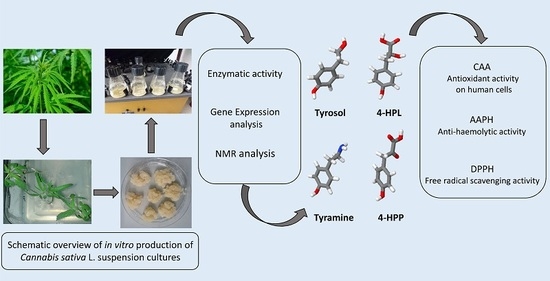
 “Use of
“Use of  “In final appraisal documents the UK National Institute for Health and Care Excellence has recommended the use of cannabidiol with clobazam for treating seizures associated with two rare and severe forms of epilepsy: Lennox-Gastaut syndrome and Dravet syndrome.
“In final appraisal documents the UK National Institute for Health and Care Excellence has recommended the use of cannabidiol with clobazam for treating seizures associated with two rare and severe forms of epilepsy: Lennox-Gastaut syndrome and Dravet syndrome. “Chronic pain is highly prevalent in most of the industrialized nations around the world. Despite the documented adverse effects, opioids are widely used for pain management.
“Chronic pain is highly prevalent in most of the industrialized nations around the world. Despite the documented adverse effects, opioids are widely used for pain management.