 “Compounds present in Cannabis sativa such as phytocannabinoids and terpenoids may act in concert to elicit therapeutic effects. Cannabinoids such as Δ9-tetrahydrocannabinol (Δ9-THC) directly activate cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2); however, it is not known if terpenoids present in Cannabis also affect cannabinoid receptor signaling. Therefore, we examined six common terpenoids alone, and in combination with cannabinoid receptor agonists, on CB1 and CB2 signaling in vitro.
“Compounds present in Cannabis sativa such as phytocannabinoids and terpenoids may act in concert to elicit therapeutic effects. Cannabinoids such as Δ9-tetrahydrocannabinol (Δ9-THC) directly activate cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2); however, it is not known if terpenoids present in Cannabis also affect cannabinoid receptor signaling. Therefore, we examined six common terpenoids alone, and in combination with cannabinoid receptor agonists, on CB1 and CB2 signaling in vitro.
Results: α-Pinene, β-pinene, β-caryophyllene, linalool, limonene, and β-myrcene (up to 30-100 μM) did not change membrane potential in AtT20 cells expressing CB1 or CB2, or affect the response to a maximally effective concentration of the synthetic cannabinoid CP55,940. The presence of individual or a combination of terpenoids did not affect the hyperpolarization produced by Δ9-THC (10 μM): (CB1: control, 59%±7%; with terpenoids (10 μM each) 55%±4%; CB2: Δ9-THC 16%±5%, with terpenoids (10 μM each) 17%±4%). To investigate possible effect on desensitization of CB1 responses, all six terpenoids were added together with Δ9-THC and signaling measured continuously over 30 min. Terpenoids did not affect desensitization, after 30 min the control hyperpolarization recovered by 63%±6% in the presence of the terpenoids recovery was 61%±5%.
Discussion: None of the six of the most common terpenoids in Cannabis directly activated CB1 or CB2, or modulated the signaling of the phytocannabinoid agonist Δ9-THC. These results suggest that if a phytocannabinoid-terpenoid entourage effect exists, it is not at the CB1 or CB2 receptor level. It remains possible that terpenoids activate CB1 and CB2 signaling pathways that do not involve potassium channels; however, it seems more likely that they may act at different molecular target(s) in the neuronal circuits important for the behavioral effect of Cannabis.”
https://www.ncbi.nlm.nih.gov/pubmed/31559333
https://www.liebertpub.com/doi/10.1089/can.2019.0016

 “2-Arachidonoyl-glycerol (2-AG) is an
“2-Arachidonoyl-glycerol (2-AG) is an 
 “Use of medical cannabis for improving symptoms of inflammatory bowel disease is increasing. However, reports on long-term outcomes are lacking. This prospective, observational study assessed the effects of licensed cannabis use among patients with inflammatory bowel disease.
“Use of medical cannabis for improving symptoms of inflammatory bowel disease is increasing. However, reports on long-term outcomes are lacking. This prospective, observational study assessed the effects of licensed cannabis use among patients with inflammatory bowel disease. “Indisputably, cancer is a global crisis that requires immediate intervention. Despite the use of conventional treatments over the past decades, it is acceptable to admit that these are expensive, invasive, associated with many side effects and, therefore, a reduced quality of life.
“Indisputably, cancer is a global crisis that requires immediate intervention. Despite the use of conventional treatments over the past decades, it is acceptable to admit that these are expensive, invasive, associated with many side effects and, therefore, a reduced quality of life.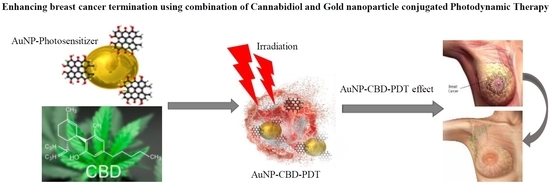
 “Patients with ulcerative colitis (UC) using marijuana have been reported to experience symptomatic benefit.
“Patients with ulcerative colitis (UC) using marijuana have been reported to experience symptomatic benefit. “Here, we hypothesized that adolescent Δ9-tetrahydrocannabinol (THC) worsens the impact of prenatal maternal immune activation (MIA) on ventral tegmental area (VTA) dopamine cells in rat offspring.
“Here, we hypothesized that adolescent Δ9-tetrahydrocannabinol (THC) worsens the impact of prenatal maternal immune activation (MIA) on ventral tegmental area (VTA) dopamine cells in rat offspring. “Cannabinoid receptors have been detected in human gliomas and cannabinoids have been proposed as novel drug candidates in the treatment of brain tumors.
“Cannabinoid receptors have been detected in human gliomas and cannabinoids have been proposed as novel drug candidates in the treatment of brain tumors. “Substance use disorder (SUD) is a major public health crisis worldwide, and effective treatment options are limited.
“Substance use disorder (SUD) is a major public health crisis worldwide, and effective treatment options are limited.