“Melanoma is one of the most aggressive malignances in human. Recently developed therapies improved overall survival rate, however, the treatment of melanoma still remains a challenging issue.
“Melanoma is one of the most aggressive malignances in human. Recently developed therapies improved overall survival rate, however, the treatment of melanoma still remains a challenging issue.
This review attempts to summarize recent advances in studies on cannabinoids used in the setting of melanoma treatment.
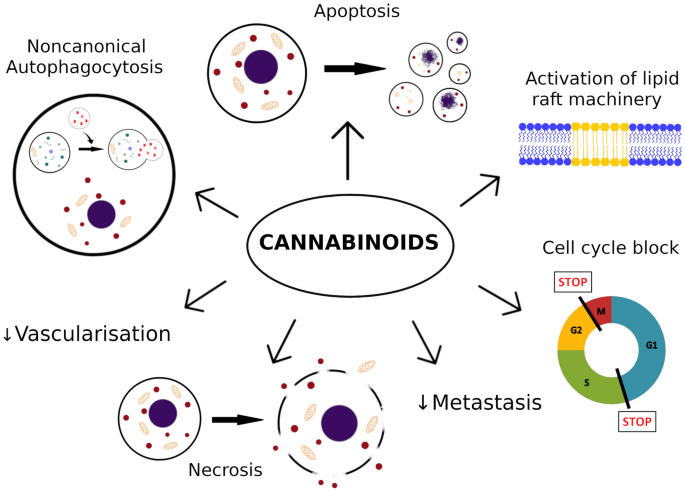
Conclusions after analysis of available data suggest that cannabinoids limit number of metastasis, and reduce growth of melanoma. The findings indicate that cannabinoids induce apoptosis, necrosis, autophagy, cell cycle arrest and exert significant interactions with tumor microenvironment.
Cannabinoids should be rather considered as a part of multi-targeted anti-tumor therapy instead of being standalone agent. Moreover, cannabinoids are likely to improve quality of life in patients with cancer, due to different supportive effects, like analgesia and/or anti-emetic effects.
In this review, it was pointed out that cannabinoids may be potentially useful in the melanoma therapy. Nevertheless, due to limited amount of data, great variety of cannabinoids available and lack of clinical trials, further studies are required to determine an exact role of cannabinoids in the treatment of melanoma.”
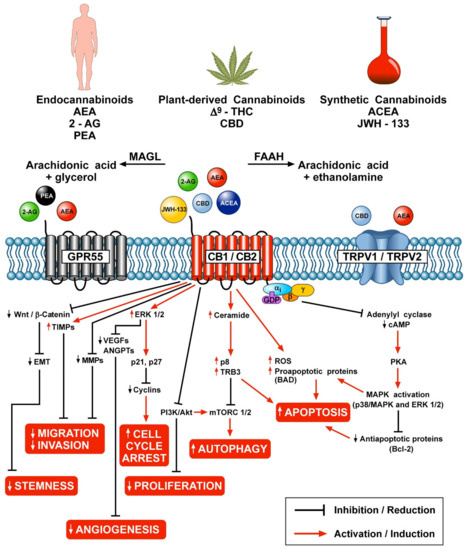
“The endocannabinoid system is dysregulated in numerous pathological conditions, including malignancies. Recently, cannabinoids have received increasing amount of interest in the setting of treatment of various cancers. Cannabinoids seem to be promising agents in the setting of melanoma treatment. In the case of melanoma, most important actions of cannabinoids described so far are decrease of cells viability by increase of apoptosis, necrosis and cell cycle arrest. Moreover, cannabinoids slow down disease progress by reduction of metastasis and tumor vascularization. Due to large variety of cannabinoids, there are many potential derivatives, which may be found useful in the therapy of melanoma.”
https://link.springer.com/article/10.1007%2Fs43440-021-00308-1

 “The endocannabinoid system (ECS) is a composite cell-signaling system that allows endogenous cannabinoid ligands to control cell functions through the interaction with cannabinoid receptors. Modifications of the ECS might contribute to the pathogenesis of different diseases, including cancers. However, the use of these compounds as antitumor agents remains debatable.
“The endocannabinoid system (ECS) is a composite cell-signaling system that allows endogenous cannabinoid ligands to control cell functions through the interaction with cannabinoid receptors. Modifications of the ECS might contribute to the pathogenesis of different diseases, including cancers. However, the use of these compounds as antitumor agents remains debatable. 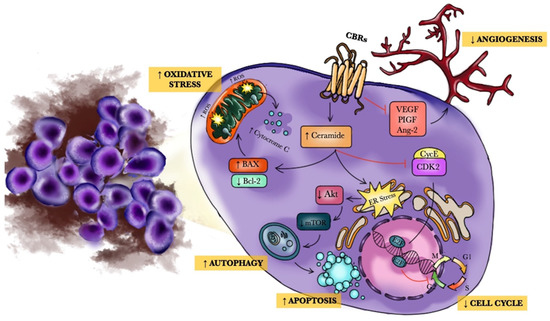
 “Medical marijuana (MM) use is common among cancer patients, but relatively little is known about the usage patterns and efficacy of MM used by gynecologic cancer patients.
“Medical marijuana (MM) use is common among cancer patients, but relatively little is known about the usage patterns and efficacy of MM used by gynecologic cancer patients. “Few studies have examined the dose-response and temporal relationships between marijuana use and ischemic stroke while controlling for important confounders, including the amount of tobacco smoking. The purpose of our study was to address these knowledge gaps.
“Few studies have examined the dose-response and temporal relationships between marijuana use and ischemic stroke while controlling for important confounders, including the amount of tobacco smoking. The purpose of our study was to address these knowledge gaps. “Human immunodeficiency virus (HIV) infection and antiretroviral therapy can independently induce HIV-associated neuropathic pain (HIV-NP).
“Human immunodeficiency virus (HIV) infection and antiretroviral therapy can independently induce HIV-associated neuropathic pain (HIV-NP). “Recent cannabis exposure has been associated with lower rates of neurocognitive impairment in people with HIV (PWH). Cannabis’s anti-inflammatory properties may underlie this relationship by reducing chronic neuroinflammation in PWH.
“Recent cannabis exposure has been associated with lower rates of neurocognitive impairment in people with HIV (PWH). Cannabis’s anti-inflammatory properties may underlie this relationship by reducing chronic neuroinflammation in PWH.  “This study aimed to obtain and characterize extracted hemp oil enriched in cannabidiol (CBD) by decarboxylation of cannabidiolic acid (CBDA) and to give new insights into its antioxidant and anticancer effects.
“This study aimed to obtain and characterize extracted hemp oil enriched in cannabidiol (CBD) by decarboxylation of cannabidiolic acid (CBDA) and to give new insights into its antioxidant and anticancer effects. 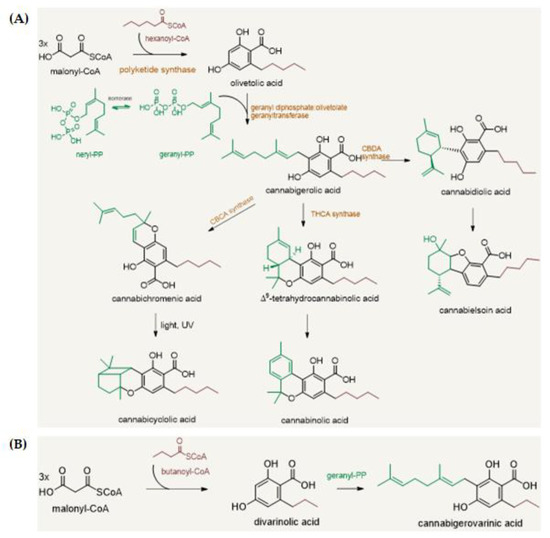
 “The therapeutic potential of
“The therapeutic potential of