 “The therapeutic potential of Cannabis sativa has been recognized since ancient times. Phytocannabinoids, endocannabinoids and synthetic cannabinoids activate two major G protein-coupled receptors, subtype 1 and 2 (CB1 and CB2). Cannabinoids (CBs) modulate several aspects of cancer cells, such as apoptosis, autophagy, proliferation, migration, epithelial-to-mesenchymal transition and stemness. Moreover, agonists of CB1 and CB2 receptors inhibit angiogenesis and lymphangiogenesis in vitro and in vivo. Low-grade inflammation is a hallmark of cancer in the tumor microenvironment (TME), which contains a plethora of innate and adaptive immune cells. These cells play a central role in tumor initiation and growth and the formation of metastasis. CB2 and, to a lesser extent, CB1 receptors are expressed on a variety of immune cells present in TME (e.g., T cells, macrophages, mast cells, neutrophils, NK cells, dendritic cells, monocytes, eosinophils). The activation of CB receptors modulates a variety of biological effects on cells of the adaptive and innate immune system. The expression of CB2 and CB1 on different subsets of immune cells in TME and hence in tumor development is incompletely characterized. The recent characterization of the human cannabinoid receptor CB2-Gi signaling complex will likely aid to design potent and specific CB2/CB1 ligands with therapeutic potential in cancer.”
“The therapeutic potential of Cannabis sativa has been recognized since ancient times. Phytocannabinoids, endocannabinoids and synthetic cannabinoids activate two major G protein-coupled receptors, subtype 1 and 2 (CB1 and CB2). Cannabinoids (CBs) modulate several aspects of cancer cells, such as apoptosis, autophagy, proliferation, migration, epithelial-to-mesenchymal transition and stemness. Moreover, agonists of CB1 and CB2 receptors inhibit angiogenesis and lymphangiogenesis in vitro and in vivo. Low-grade inflammation is a hallmark of cancer in the tumor microenvironment (TME), which contains a plethora of innate and adaptive immune cells. These cells play a central role in tumor initiation and growth and the formation of metastasis. CB2 and, to a lesser extent, CB1 receptors are expressed on a variety of immune cells present in TME (e.g., T cells, macrophages, mast cells, neutrophils, NK cells, dendritic cells, monocytes, eosinophils). The activation of CB receptors modulates a variety of biological effects on cells of the adaptive and innate immune system. The expression of CB2 and CB1 on different subsets of immune cells in TME and hence in tumor development is incompletely characterized. The recent characterization of the human cannabinoid receptor CB2-Gi signaling complex will likely aid to design potent and specific CB2/CB1 ligands with therapeutic potential in cancer.”
https://pubmed.ncbi.nlm.nih.gov/34064197/
https://www.mdpi.com/2073-4409/10/6/1282
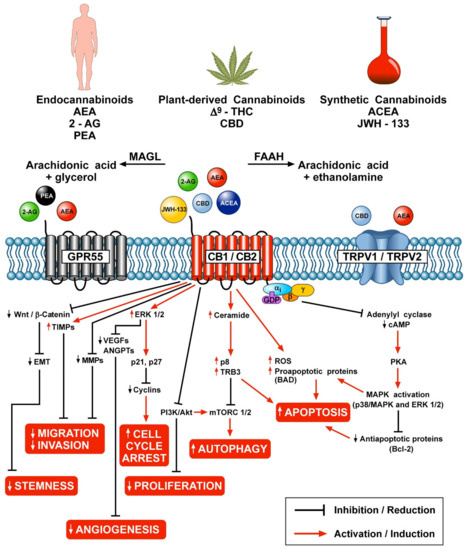

 “Cannabinoids, including delta-8- and delta-9-tetrahydrocannabinol (THC) have a palliative care impact and may therefore be beneficial against cancer.
“Cannabinoids, including delta-8- and delta-9-tetrahydrocannabinol (THC) have a palliative care impact and may therefore be beneficial against cancer.  “Background:
“Background:  “Introduction:
“Introduction: “The cannabinoid, cannabidiol (CBD), is part of the plant’s natural defense system that when given to animals has many useful medicinal properties, including activity against cancer cells, modulation of the immune system, and efficacy in epilepsy.
“The cannabinoid, cannabidiol (CBD), is part of the plant’s natural defense system that when given to animals has many useful medicinal properties, including activity against cancer cells, modulation of the immune system, and efficacy in epilepsy.  “Venous Leg Ulcers are highly prevalent lower limb integumentary wounds that remain challenging to heal despite the use of evidence-based compression therapies. A multitude of adjuvant treatments have been studied but none have demonstrated enough efficacy to gain adoption into treatment guidelines.
“Venous Leg Ulcers are highly prevalent lower limb integumentary wounds that remain challenging to heal despite the use of evidence-based compression therapies. A multitude of adjuvant treatments have been studied but none have demonstrated enough efficacy to gain adoption into treatment guidelines.  “In recent decades, epidemiological, clinical, and experimental studies have demonstrated that a diet with antioxidant or anti-inflammatory function plays a central role in the prevention of atherosclerosis (AS).
“In recent decades, epidemiological, clinical, and experimental studies have demonstrated that a diet with antioxidant or anti-inflammatory function plays a central role in the prevention of atherosclerosis (AS).  “Cannabinoids such as ▵-9-THC and CBD can downregulate the immune response by modulating the endocannabinoid system. This modulation is relevant for the treatment of prevalent autoimmune diseases (ADs), such as multiple sclerosis (MS), systemic lupus erythematosus (SLE), diabetes mellitus type 1 (DMT1), and rheumatoid arthritis (RA). These conditions require new therapeutic options with fewer side effects for the control of the autoimmune response. Objective: to conduct a literature review of preclinical scientific evidence that supports further clinical investigations for the use of cannabinoids (natural or synthetic) as potential immunomodulators of the immune response in ADs.
“Cannabinoids such as ▵-9-THC and CBD can downregulate the immune response by modulating the endocannabinoid system. This modulation is relevant for the treatment of prevalent autoimmune diseases (ADs), such as multiple sclerosis (MS), systemic lupus erythematosus (SLE), diabetes mellitus type 1 (DMT1), and rheumatoid arthritis (RA). These conditions require new therapeutic options with fewer side effects for the control of the autoimmune response. Objective: to conduct a literature review of preclinical scientific evidence that supports further clinical investigations for the use of cannabinoids (natural or synthetic) as potential immunomodulators of the immune response in ADs.