 “Neuropathic pain conditions including neuropathic orofacial pain (NOP) are difficult to treat. Contemporary therapeutic agents for neuropathic pain are often ineffective in relieving pain and are associated with various adverse effects. Finding new options for treating neuropathic pain is a major priority in pain-related research.
“Neuropathic pain conditions including neuropathic orofacial pain (NOP) are difficult to treat. Contemporary therapeutic agents for neuropathic pain are often ineffective in relieving pain and are associated with various adverse effects. Finding new options for treating neuropathic pain is a major priority in pain-related research.
Cannabinoid-based therapeutic strategies have emerged as promising new options.
Cannabinoids mainly act on cannabinoid 1 (CB1) and 2 (CB2) receptors, and the former is widely distributed in the brain. The therapeutic significance of cannabinoids is masked by their adverse effects including sedation, motor impairment, addiction and cognitive impairment, which are thought to be mediated by CB1 receptors in the brain. Alternative approaches have been developed to overcome this problem by selectively targeting CB2 receptors, peripherally restricted CB1 receptors and endocannabinoids that may be locally synthesized on demand at sites where their actions are pertinent.
Many preclinical studies have reported that these strategies are effective for treating neuropathic pain and produce no or minimal side effects.
Recently, we observed that inhibition of degradation of a major endocannabinoid, 2-arachydonoylglycerol, can attenuate NOP following trigeminal nerve injury in mice. This review will discuss the above-mentioned alternative approaches that show potential for treating neuropathic pain including NOP.”

 “Metastatic breast cancer is prevalent worldwide, and one of the most common sites of metastasis are long bones. Of patients with disease, the major symptom is pain, yet current medications fail to adequately result in analgesic efficacy and present major undesirable adverse effects.
“Metastatic breast cancer is prevalent worldwide, and one of the most common sites of metastasis are long bones. Of patients with disease, the major symptom is pain, yet current medications fail to adequately result in analgesic efficacy and present major undesirable adverse effects. “Patients with non-small cell lung cancer (NSCLC) develop resistance to antitumor agents by mechanisms that involve the epithelial-to-mesenchymal transition (EMT). This necessitates the development of new complementary drugs, e.g.,
“Patients with non-small cell lung cancer (NSCLC) develop resistance to antitumor agents by mechanisms that involve the epithelial-to-mesenchymal transition (EMT). This necessitates the development of new complementary drugs, e.g.,  “Cannabinoids are a group of structurally heterogeneous but pharmacologically related compounds, including plant-derived cannabinoids, synthetic substances and endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol.
“Cannabinoids are a group of structurally heterogeneous but pharmacologically related compounds, including plant-derived cannabinoids, synthetic substances and endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol. “Most of the drugs of abuse affect the brain by interacting with naturally expressed molecular receptors. Marihuana affects a series of receptors including
“Most of the drugs of abuse affect the brain by interacting with naturally expressed molecular receptors. Marihuana affects a series of receptors including  “Human adipose tissue includes large quantities of mesenchymal stromal cells (atMSCs), which represent an abundant cell source for therapeutic applications in the field of regenerative medicine.
“Human adipose tissue includes large quantities of mesenchymal stromal cells (atMSCs), which represent an abundant cell source for therapeutic applications in the field of regenerative medicine.
 “Drugs selectively targeting CB2 hold promise for treating neurodegenerative disorders, inflammation, and pain while avoiding psychotropic side effects mediated by CB1. The mechanisms underlying CB2 activation and signaling are poorly understood but critical for drug design. Here we report the cryo-EM structure of the human CB2-Gi signaling complex bound to the agonist WIN 55,212-2. The 3D structure reveals the binding mode of WIN 55,212-2 and structural determinants for distinguishing CB2 agonists from antagonists, which are supported by a pair of rationally designed agonist and antagonist. Further structural analyses with computational docking results uncover the differences between CB2 and CB1 in receptor activation, ligand recognition, and Gi coupling. These findings are expected to facilitate rational structure-based discovery of drugs targeting the
“Drugs selectively targeting CB2 hold promise for treating neurodegenerative disorders, inflammation, and pain while avoiding psychotropic side effects mediated by CB1. The mechanisms underlying CB2 activation and signaling are poorly understood but critical for drug design. Here we report the cryo-EM structure of the human CB2-Gi signaling complex bound to the agonist WIN 55,212-2. The 3D structure reveals the binding mode of WIN 55,212-2 and structural determinants for distinguishing CB2 agonists from antagonists, which are supported by a pair of rationally designed agonist and antagonist. Further structural analyses with computational docking results uncover the differences between CB2 and CB1 in receptor activation, ligand recognition, and Gi coupling. These findings are expected to facilitate rational structure-based discovery of drugs targeting the 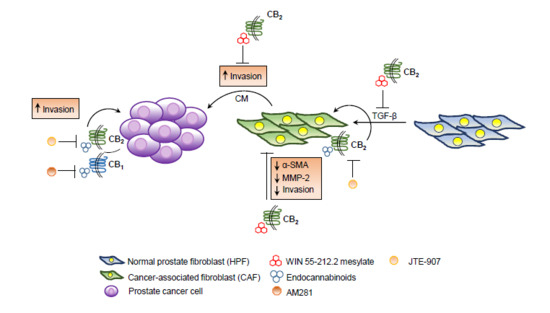
 “Parkinson’s disease is a neurodegenerative disorder, the motor symptoms of which are associated classically with Lewy body formation and nigrostriatal degeneration.
“Parkinson’s disease is a neurodegenerative disorder, the motor symptoms of which are associated classically with Lewy body formation and nigrostriatal degeneration.