 “Cannabis sativa and its principal components, Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol, are increasingly being used to treat a variety of medical problems, including inflammatory conditions.
“Cannabis sativa and its principal components, Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol, are increasingly being used to treat a variety of medical problems, including inflammatory conditions.
Although studies suggest that the endocannabinoid system has immunomodulatory properties, there remains a paucity of information on the effects of cannabinoids on immunity and on outcomes of infection and injury.
We investigated the effects and mechanism(s) of action of cannabinoid receptor agonists, including Δ9-THC, on inflammation and organ injury in endotoxemic mice.
Administration of Δ9-THC caused a dramatic early upregulation of plasma IL-10 levels, reduced plasma IL-6 and CCL-2 levels, led to better clinical status, and attenuated organ injury in endotoxemic mice. The anti-inflammatory effects of Δ9-THC in endotoxemic mice were reversed by a cannabinoid receptor type 1 (CB1R) inverse agonist (SR141716), and by clodronate-induced myeloid-cell depletion, but not by genetic invalidation or blockade of other putative Δ9-THC receptors, including cannabinoid receptor type 2, TRPV1, GPR18, GPR55, and GPR119. Although Δ9-THC administration reduced the activation of several spleen immune cell subsets, the anti-inflammatory effects of Δ9-THC were preserved in splenectomized endotoxemic mice. Finally, using IL-10-GFP reporter mice, we showed that blood monocytic myeloid-derived suppressive cells mediate the Δ9-THC-induced early rise in circulating IL-10.
These results indicate that Δ9-THC potently induces IL-10, while reducing proinflammatory cytokines, chemokines, and related organ injury in endotoxemic mice via the activation of CB1R. These data have implications for acute and chronic conditions that are driven by dysregulated inflammation, such as sepsis, and raise the possibility that CB1R-signaling may constitute a novel target for inflammatory disorders.”
https://www.ncbi.nlm.nih.gov/pubmed/32385136
https://www.jimmunol.org/content/early/2020/05/07/jimmunol.2000213
 “The inhibitor of DNA binding (Id) proteins are regulators of cell cycle and cell differentiation. Of all Id family proteins, Id1 is mostly linked to tumorigenesis, cellular senescence as well as cell proliferation and survival.
“The inhibitor of DNA binding (Id) proteins are regulators of cell cycle and cell differentiation. Of all Id family proteins, Id1 is mostly linked to tumorigenesis, cellular senescence as well as cell proliferation and survival.
 “There is increasing interest in the use of purified
“There is increasing interest in the use of purified 
 “The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.
“The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.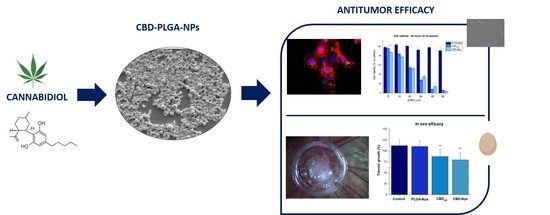
 “Cannabis sativa and its principal components, Δ9-tetrahydrocannabinol (Δ9-THC) and
“Cannabis sativa and its principal components, Δ9-tetrahydrocannabinol (Δ9-THC) and 

 “The prevalence rates of depression and anxiety are at least two times higher in diabetic patients, increasing morbidity and mortality.
“The prevalence rates of depression and anxiety are at least two times higher in diabetic patients, increasing morbidity and mortality. “Gestational methylazoxymethanol acetate (MAM) treatment produces offspring with adult phenotype relevant to schizophrenia, including positive- and negative-like symptoms, cognitive deficits, dopaminergic dysfunction, structural and functional abnormalities.
“Gestational methylazoxymethanol acetate (MAM) treatment produces offspring with adult phenotype relevant to schizophrenia, including positive- and negative-like symptoms, cognitive deficits, dopaminergic dysfunction, structural and functional abnormalities.
 “Cannabis sativa is an aromatic annual flowering plant with several botanical varieties, used for different purposes, like the production of fibers, the production of oil from the seeds, and especially for recreational or medical purposes.
“Cannabis sativa is an aromatic annual flowering plant with several botanical varieties, used for different purposes, like the production of fibers, the production of oil from the seeds, and especially for recreational or medical purposes.
 “Borderline Personality Disorder (BPD) is a chronic debilitating psychiatric disorder characterized mainly by emotional instability, chaotic interpersonal relationships, cognitive disturbance (e.g. dissociation and suicidal thoughts) and maladaptive behaviors. BPD has a high rate of comorbidity with other mental disorders and high burden on society.
“Borderline Personality Disorder (BPD) is a chronic debilitating psychiatric disorder characterized mainly by emotional instability, chaotic interpersonal relationships, cognitive disturbance (e.g. dissociation and suicidal thoughts) and maladaptive behaviors. BPD has a high rate of comorbidity with other mental disorders and high burden on society.