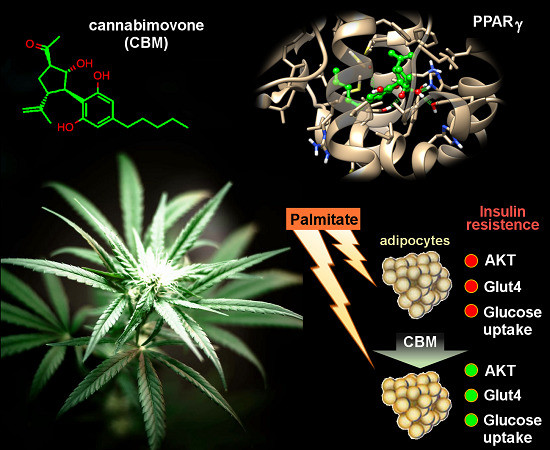
 “Phytocannabinoids (pCBs) are a large family of meroterpenoids isolated from the plant Cannabis sativa. Δ9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best investigated phytocannabinoids due to their relative abundance and interesting bioactivity profiles. In addition to various targets, THC and CBD are also well-known agonists of peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear receptor involved in energy homeostasis and lipid metabolism. In the search of new pCBs potentially acting as PPARγ agonists, we identified cannabimovone (CBM), a structurally unique abeo-menthane pCB, as a novel PPARγ modulator via a combined computational and experimental approach. The ability of CBM to act as dual PPARγ/α agonist was also evaluated. Computational studies suggested a different binding mode toward the two isoforms, with the compound able to recapitulate the pattern of H-bonds of a canonical agonist only in the case of PPARγ. Luciferase assays confirmed the computational results, showing a selective activation of PPARγ by CBM in the low micromolar range. CBM promoted the expression of PPARγ target genes regulating the adipocyte differentiation and prevented palmitate-induced insulin signaling impairment. Altogether, these results candidate CBM as a novel bioactive compound potentially useful for the treatment of insulin resistance-related disorders.”
“Phytocannabinoids (pCBs) are a large family of meroterpenoids isolated from the plant Cannabis sativa. Δ9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best investigated phytocannabinoids due to their relative abundance and interesting bioactivity profiles. In addition to various targets, THC and CBD are also well-known agonists of peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear receptor involved in energy homeostasis and lipid metabolism. In the search of new pCBs potentially acting as PPARγ agonists, we identified cannabimovone (CBM), a structurally unique abeo-menthane pCB, as a novel PPARγ modulator via a combined computational and experimental approach. The ability of CBM to act as dual PPARγ/α agonist was also evaluated. Computational studies suggested a different binding mode toward the two isoforms, with the compound able to recapitulate the pattern of H-bonds of a canonical agonist only in the case of PPARγ. Luciferase assays confirmed the computational results, showing a selective activation of PPARγ by CBM in the low micromolar range. CBM promoted the expression of PPARγ target genes regulating the adipocyte differentiation and prevented palmitate-induced insulin signaling impairment. Altogether, these results candidate CBM as a novel bioactive compound potentially useful for the treatment of insulin resistance-related disorders.”
https://www.ncbi.nlm.nih.gov/pubmed/32138197
https://www.mdpi.com/1420-3049/25/5/1119

 “Cannabinoids are extracted from Cannabis sativa L. and are used for a variety of medicinal purposes.
“Cannabinoids are extracted from Cannabis sativa L. and are used for a variety of medicinal purposes. “Psoriasis is a chronic inflammatory skin disease characterized by dysregulated keratinocyte differentiation, but oxidative stress also plays an important role in the pathogenesis of this disease.
“Psoriasis is a chronic inflammatory skin disease characterized by dysregulated keratinocyte differentiation, but oxidative stress also plays an important role in the pathogenesis of this disease. “Multiple sclerosis (MS) is a highly symptomatic disease, with a wide range of disabilities affecting many bodily functions, even in younger persons with a short disease history.
“Multiple sclerosis (MS) is a highly symptomatic disease, with a wide range of disabilities affecting many bodily functions, even in younger persons with a short disease history. “In vivo studies show that
“In vivo studies show that  “Clinical evidence supports effectiveness of
“Clinical evidence supports effectiveness of  “Alzheimer’s disease (AD) is characterized by progressive cognitive decline and pathologically by the accumulation of amyloid-β (Aβ) and tau hyperphosphorylation causing neurodegeneration and neuroinflammation. Current AD treatments do not stop or reverse the disease progression, highlighting the need for more effective therapeutics.
“Alzheimer’s disease (AD) is characterized by progressive cognitive decline and pathologically by the accumulation of amyloid-β (Aβ) and tau hyperphosphorylation causing neurodegeneration and neuroinflammation. Current AD treatments do not stop or reverse the disease progression, highlighting the need for more effective therapeutics. “Herein, 11 general types of natural cannabinoids from Cannabis sativa as well as 50 (-)-CBD analogues with therapeutic potential were described. The underlying molecular mechanisms of CBD as a therapeutic candidate for epilepsy and neurodegenerative diseases were comprehensively clarified. CBD indirectly acts as an endogenous cannabinoid receptor agonist to exert its neuroprotective effects. CBD also promotes neuroprotection through different signal transduction pathways mediated indirectly by cannabinoid receptors. Furthermore, CBD prevents the glycogen synthase kinase 3β (GSK-3β) hyperphosphorylation caused by Aβ and may be developed as a new therapeutic candidate for Alzheimer’s disease.”
“Herein, 11 general types of natural cannabinoids from Cannabis sativa as well as 50 (-)-CBD analogues with therapeutic potential were described. The underlying molecular mechanisms of CBD as a therapeutic candidate for epilepsy and neurodegenerative diseases were comprehensively clarified. CBD indirectly acts as an endogenous cannabinoid receptor agonist to exert its neuroprotective effects. CBD also promotes neuroprotection through different signal transduction pathways mediated indirectly by cannabinoid receptors. Furthermore, CBD prevents the glycogen synthase kinase 3β (GSK-3β) hyperphosphorylation caused by Aβ and may be developed as a new therapeutic candidate for Alzheimer’s disease.”
 “The 20% prevalence of chronic pain in the general population is a major health concern given the often profound associated impairment of daily activities, employment status, and health-related quality of life in sufferers. Resource utilization associated with chronic pain represents an enormous burden for healthcare systems. Although analgesia based on the World Health Organization’s pain ladder continues to be the mainstay of chronic pain management, aside from chronic cancer pain or end-of-life care, prolonged use of non-steroidal anti-inflammatory drugs or opioids to manage chronic pain is rarely sustainable.
“The 20% prevalence of chronic pain in the general population is a major health concern given the often profound associated impairment of daily activities, employment status, and health-related quality of life in sufferers. Resource utilization associated with chronic pain represents an enormous burden for healthcare systems. Although analgesia based on the World Health Organization’s pain ladder continues to be the mainstay of chronic pain management, aside from chronic cancer pain or end-of-life care, prolonged use of non-steroidal anti-inflammatory drugs or opioids to manage chronic pain is rarely sustainable.