 “This study attempted to provide the effects and mechanisms of two cannabinoids, O-1602 and cannabidiol (CBD), on colonic motility of 2,4,6-trinitro-benzene sulfonic acid (TNBS) colitis.
“This study attempted to provide the effects and mechanisms of two cannabinoids, O-1602 and cannabidiol (CBD), on colonic motility of 2,4,6-trinitro-benzene sulfonic acid (TNBS) colitis.
METHODS:
TNBS was used to induce the model of motility disorder. G protein-coupled receptor 55 (GPR55) expression was detected using real-time PCR and immunohistochemistry in colon. Pro-inflammatory cytokines and myeloperoxidase were also measured. The colonic motility was measured by upper GI transit in vivo and recorded using electrical stimulation organ bath technique in vitro. Freshly isolated smooth muscle from the rat colon were applied to determine the membrane potential and Ca2+ -ATPase activity, respectively.
KEY RESULTS:
CBD or O-1602 separately improved inflammatory conditions significantly in TNBS-induced colitis rats. However, sole CBD pretreatment reduced GPR55 expression, which was up-regulated in TNBS colitis. O-1602 and CBD each lowered MPO and IL-6 levels remarkably in TNBS colitis, while TNF-α levels experienced no change. CBD rescued the downward colonic motility in TNBS colitis in vivo; however, it decreased the upward contraction of the smooth muscle strip under electrical stimulation in vitro. Pretreatment with CBD prevented against TNBS-induced changes of Ca2+ -ATPase activity of smooth muscle cells. However, membrane potential of the smooth muscle cells decreased by TNBS experienced no change after O-1602 or CBD import.
CONCLUSIONS & INFERENCES:
The present study suggested that CBD participated in the regulation of colonic motility in rats, and the mechanisms may be involved in the regulation of inlammatory factors and Ca2+ -ATPase activity through GPR55.”


 “Inflammation is a common feature of many neurodegenerative diseases.
“Inflammation is a common feature of many neurodegenerative diseases.
 “There is considerable interest in the use of cannabinoids for symptom control in palliative care, but there is little high-quality evidence to guide clinical practice.
“There is considerable interest in the use of cannabinoids for symptom control in palliative care, but there is little high-quality evidence to guide clinical practice. “A 64 year old male heating engineer was investigated for a persistent cough and found to have epithelioid mesothelioma with pleural effusion, lung nodules and increased thoracic lymph nodes. He declined standard of care treatment following his own research and he was enrolled in a named patient programme of IMM-101. He was advised to correct his low vitamin D3 level and to start using anti-inflammatories such as aspirin, bromelain and low dose Naltrexone. At review one year later a CT scan showed no change and he continued on the regimen. Four years after the diagnosis a CT scan showed that there was a modest but definite progression of the left malignant pleural thickening, and a new right-sided effusion, enlargement of several intrathoracic nodes which had been noted on the early scans. The chest wall lump eventually broke down and required local radiotherapy. He then developed abdominal pain and found to have peritoneal disease. Last year he obtained the
“A 64 year old male heating engineer was investigated for a persistent cough and found to have epithelioid mesothelioma with pleural effusion, lung nodules and increased thoracic lymph nodes. He declined standard of care treatment following his own research and he was enrolled in a named patient programme of IMM-101. He was advised to correct his low vitamin D3 level and to start using anti-inflammatories such as aspirin, bromelain and low dose Naltrexone. At review one year later a CT scan showed no change and he continued on the regimen. Four years after the diagnosis a CT scan showed that there was a modest but definite progression of the left malignant pleural thickening, and a new right-sided effusion, enlargement of several intrathoracic nodes which had been noted on the early scans. The chest wall lump eventually broke down and required local radiotherapy. He then developed abdominal pain and found to have peritoneal disease. Last year he obtained the  “Peripheral neuropathy can significantly impact the quality of life for those who are affected, as therapies from the current treatment algorithm often fail to deliver adequate symptom relief. There has, however, been an increasing body of evidence for the use of cannabinoids in the treatment of chronic, noncancer pain. The efficacy of a topically delivered
“Peripheral neuropathy can significantly impact the quality of life for those who are affected, as therapies from the current treatment algorithm often fail to deliver adequate symptom relief. There has, however, been an increasing body of evidence for the use of cannabinoids in the treatment of chronic, noncancer pain. The efficacy of a topically delivered  “Accumulated evidence indicates that
“Accumulated evidence indicates that 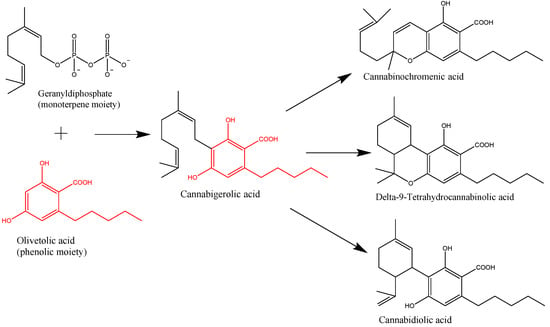
 “The monocyclic 1,4-benzoquinone, HU-331, the direct oxidation product of
“The monocyclic 1,4-benzoquinone, HU-331, the direct oxidation product of