“Phytocannabinoids are unique terpenophenolic compounds predominantly produced in the glandular trichomes of the cannabis plant (Cannabis sativa L.). The delta-9- tetrahydrocannabinol (THC) is the main active constituent responsible for the plant’s psychoactive effect and, together with the non- psychoactive cannabidiol (CBD), the most investigated naturally occurring cannabinoid.
“Phytocannabinoids are unique terpenophenolic compounds predominantly produced in the glandular trichomes of the cannabis plant (Cannabis sativa L.). The delta-9- tetrahydrocannabinol (THC) is the main active constituent responsible for the plant’s psychoactive effect and, together with the non- psychoactive cannabidiol (CBD), the most investigated naturally occurring cannabinoid.
The first report on the antitumor properties of cannabis compounds appeared more than forty years ago, but the potential of targeting the endocannabinoid system in cancer has recently attracted increasing interest. Our study aimed to review the last decade’s findings on the anticancer potential of plant- derived cannabinoids and the possible mechanisms of their activity.
A large body of in vitro data has been accumulated demonstrating that phytocannabinoids affect a wide spectrum of tumor cells, including gliomas, neuroblastomas, hepatocarcinoma as well as skin, prostate, breast, cervical, colon, pancreatic, lung and hematological cancer.
It has been found that they can stop the uncontrolled growth of cancer cells through the cell-cycle arrest, inhibition of cell proliferation and induction of autophagy and apoptosis. They can also block all the steps of tumor progression, including tumor cell migration, adhesion and invasion as well as angiogenesis. The observed effects are mainly mediated by the cannabinoid CB1 and/or CB2 receptors, although some other receptors and mechanisms unrelated to receptor stimulation may also be involved.
The majority of available animal studies confirmed that phytocannabinoids are capable of effectively decreasing cancer growth and metastasis in vivo. THC was found to be effective against experimental glioma, liver, pancreatic, breast and lung cancer while CBD showed activity against glioma and neuroblastoma, melanoma, colon, breast, prostate and lung cancer. Further in vitro and in vivo studies also greatly support their use in combination with traditional chemotherapy or radiotherapy, which results in improved efficiency, attenuated toxicity or reduced drug resistance.
Taken together most of available preclinical results emphasize the extensive therapeutic potential of THC and CBD in various types of cancers. The potential clinical interest of cannabinoids is additionally suggested by their selectivity for tumor cells as well as their good tolerance and the absence of normal tissue toxicity, which are still the major limitations of most conventional drugs. The accumulated preclinical evidence strongly suggests the need for clinical testing of cannabinoids in cancer patients.”

 “Among the assisted reproductive techniques, the in vitro maturation of oocytes (IVM) is less developed than other techniques, but its implementation would entail a qualitative advance.
“Among the assisted reproductive techniques, the in vitro maturation of oocytes (IVM) is less developed than other techniques, but its implementation would entail a qualitative advance. “The prior medical literature offers little guidance as to how pain relief and side effect manifestation may vary across commonly used and commercially available cannabis product types. We used the largest dataset in the United States of real-time responses to and side effect reporting from patient-directed cannabis consumption sessions for the treatment of pain under naturalistic conditions in order to identify how cannabis affects momentary pain intensity levels and which product characteristics are the best predictors of therapeutic pain relief.
“The prior medical literature offers little guidance as to how pain relief and side effect manifestation may vary across commonly used and commercially available cannabis product types. We used the largest dataset in the United States of real-time responses to and side effect reporting from patient-directed cannabis consumption sessions for the treatment of pain under naturalistic conditions in order to identify how cannabis affects momentary pain intensity levels and which product characteristics are the best predictors of therapeutic pain relief. “Δ9-Tetrahydrocannabinol (THC, a CB1 receptor agonist) and Cannabidiol (CBD, a non-competitive antagonist of endogenous CB1 and CB2 ligands) are two primary components of Cannabis species, and may modulate fear learning in mammals.
“Δ9-Tetrahydrocannabinol (THC, a CB1 receptor agonist) and Cannabidiol (CBD, a non-competitive antagonist of endogenous CB1 and CB2 ligands) are two primary components of Cannabis species, and may modulate fear learning in mammals. “Cannabis was extensively utilized for its medicinal properties till the 19th century. A steep decline in its medicinal usage was observed later due to its emergence as an illegal recreational drug.
“Cannabis was extensively utilized for its medicinal properties till the 19th century. A steep decline in its medicinal usage was observed later due to its emergence as an illegal recreational drug.
 “Thanks to the success of modern antiretroviral therapy (ART), people living with HIV (PLWH) have life expectancies which approach that of persons in the general population. However, despite the ability of ART to suppress viral replication, PLWH have high levels of chronic systemic inflammation which drives the development of comorbidities such as cardiovascular disease, diabetes and non-AIDS associated malignancies.
“Thanks to the success of modern antiretroviral therapy (ART), people living with HIV (PLWH) have life expectancies which approach that of persons in the general population. However, despite the ability of ART to suppress viral replication, PLWH have high levels of chronic systemic inflammation which drives the development of comorbidities such as cardiovascular disease, diabetes and non-AIDS associated malignancies. “Patients with multiple sclerosis spasticity (MSS) and upper limb/hand impairment who are taking 9-delta-tetrahydrocannabinol:
“Patients with multiple sclerosis spasticity (MSS) and upper limb/hand impairment who are taking 9-delta-tetrahydrocannabinol: “Cannabis is widely used in the United States with an estimated prevalence of 9.5%. Certain
“Cannabis is widely used in the United States with an estimated prevalence of 9.5%. Certain  “Currently, a combination of marijuana cannabinoids including delta-9-tetrahydrocannabinol (THC) and
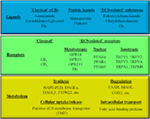
“Currently, a combination of marijuana cannabinoids including delta-9-tetrahydrocannabinol (THC) and  “It is well known that certain active ingredients of the plants of Cannabis genus, i.e., the “phytocannabinoids” [pCBs; e.g., (−)-trans-Δ9-tetrahydrocannabinol (THC), (−)-cannabidiol, etc.] can influence a wide array of biological processes, and the human body is able to produce endogenous analogs of these substances [“endocannabinoids” (eCB), e.g., arachidonoylethanolamine (anandamide, AEA), 2-arachidonoylglycerol (2-AG), etc.]. These ligands, together with multiple receptors (e.g., CB1 and CB2 cannabinoid receptors, etc.), and a complex enzyme and transporter apparatus involved in the synthesis and degradation of the ligands constitute the endocannabinoid system (ECS), a recently emerging regulator of several physiological processes. The ECS is widely expressed in the human body, including several members of the innate and adaptive immune system, where eCBs, as well as several pCBs were shown to deeply influence immune functions thereby regulating inflammation, autoimmunity, antitumor, as well as antipathogen immune responses, etc. Based on this knowledge, many in vitro and in vivo studies aimed at exploiting the putative therapeutic potential of cannabinoid signaling in inflammation-accompanied diseases (e.g., multiple sclerosis) or in organ transplantation, and to dissect the complex immunological effects of medical and “recreational” marijuana consumption. Thus, the objective of the current article is (i) to summarize the most recent findings of the field; (ii) to highlight the putative therapeutic potential of targeting cannabinoid signaling; (iii) to identify open questions and key challenges; and (iv) to suggest promising future directions for cannabinoid-based drug development.
“It is well known that certain active ingredients of the plants of Cannabis genus, i.e., the “phytocannabinoids” [pCBs; e.g., (−)-trans-Δ9-tetrahydrocannabinol (THC), (−)-cannabidiol, etc.] can influence a wide array of biological processes, and the human body is able to produce endogenous analogs of these substances [“endocannabinoids” (eCB), e.g., arachidonoylethanolamine (anandamide, AEA), 2-arachidonoylglycerol (2-AG), etc.]. These ligands, together with multiple receptors (e.g., CB1 and CB2 cannabinoid receptors, etc.), and a complex enzyme and transporter apparatus involved in the synthesis and degradation of the ligands constitute the endocannabinoid system (ECS), a recently emerging regulator of several physiological processes. The ECS is widely expressed in the human body, including several members of the innate and adaptive immune system, where eCBs, as well as several pCBs were shown to deeply influence immune functions thereby regulating inflammation, autoimmunity, antitumor, as well as antipathogen immune responses, etc. Based on this knowledge, many in vitro and in vivo studies aimed at exploiting the putative therapeutic potential of cannabinoid signaling in inflammation-accompanied diseases (e.g., multiple sclerosis) or in organ transplantation, and to dissect the complex immunological effects of medical and “recreational” marijuana consumption. Thus, the objective of the current article is (i) to summarize the most recent findings of the field; (ii) to highlight the putative therapeutic potential of targeting cannabinoid signaling; (iii) to identify open questions and key challenges; and (iv) to suggest promising future directions for cannabinoid-based drug development.