 “Deposition of amyloid-beta (Aβ) peptide in the brain is the leading source of the onset and progression of Alzheimer’s disease (AD). Recent studies have suggested that anti-amyloidogenic agents may be a suitable therapeutic strategy for AD.
“Deposition of amyloid-beta (Aβ) peptide in the brain is the leading source of the onset and progression of Alzheimer’s disease (AD). Recent studies have suggested that anti-amyloidogenic agents may be a suitable therapeutic strategy for AD.
Aim: The current review was proposed to address the beneficial effects of cannabis-based drugs for the treatment of AD, focusing primarily on Aβ modifications.
Result: A total of 17 studies were identified based on the inclusion criteria; however, nine studies qualified for this systematic review. The maximum and minimum cannabis dosages, mostly CBD and THC in animal studies, were 0.75 and 50 mg/kg, respectively. Cannabis (CBD and THC) was injected for 10 to 21 days. The findings of the 9 articles indicated that cannabis-based drugs might modulate Aβ modifications in several AD models.
Conclusion: Our findings establish that cannabis-based drugs inhibited the progression of AD by modulating Aβ modifications.”

 “Objectives: To investigate the action of cannabinoids on spasticity and pain in secondary progressive multiple sclerosis, by means of neurophysiological indexes.
“Objectives: To investigate the action of cannabinoids on spasticity and pain in secondary progressive multiple sclerosis, by means of neurophysiological indexes. “Hempseeds, the edible fruits of the Cannabis sativa L. plant, were initially considered a by-product of the hemp technical fibre industry. Nowadays, following the restorationing of the cultivation of C. sativa L. plants containing an amount of delta-9-tetrahydrocannabinol (THC) <0.3% or 0.2% (industrial hemp) there is a growing interest for the hempseeds production due to their high nutritional value and functional features.
“Hempseeds, the edible fruits of the Cannabis sativa L. plant, were initially considered a by-product of the hemp technical fibre industry. Nowadays, following the restorationing of the cultivation of C. sativa L. plants containing an amount of delta-9-tetrahydrocannabinol (THC) <0.3% or 0.2% (industrial hemp) there is a growing interest for the hempseeds production due to their high nutritional value and functional features.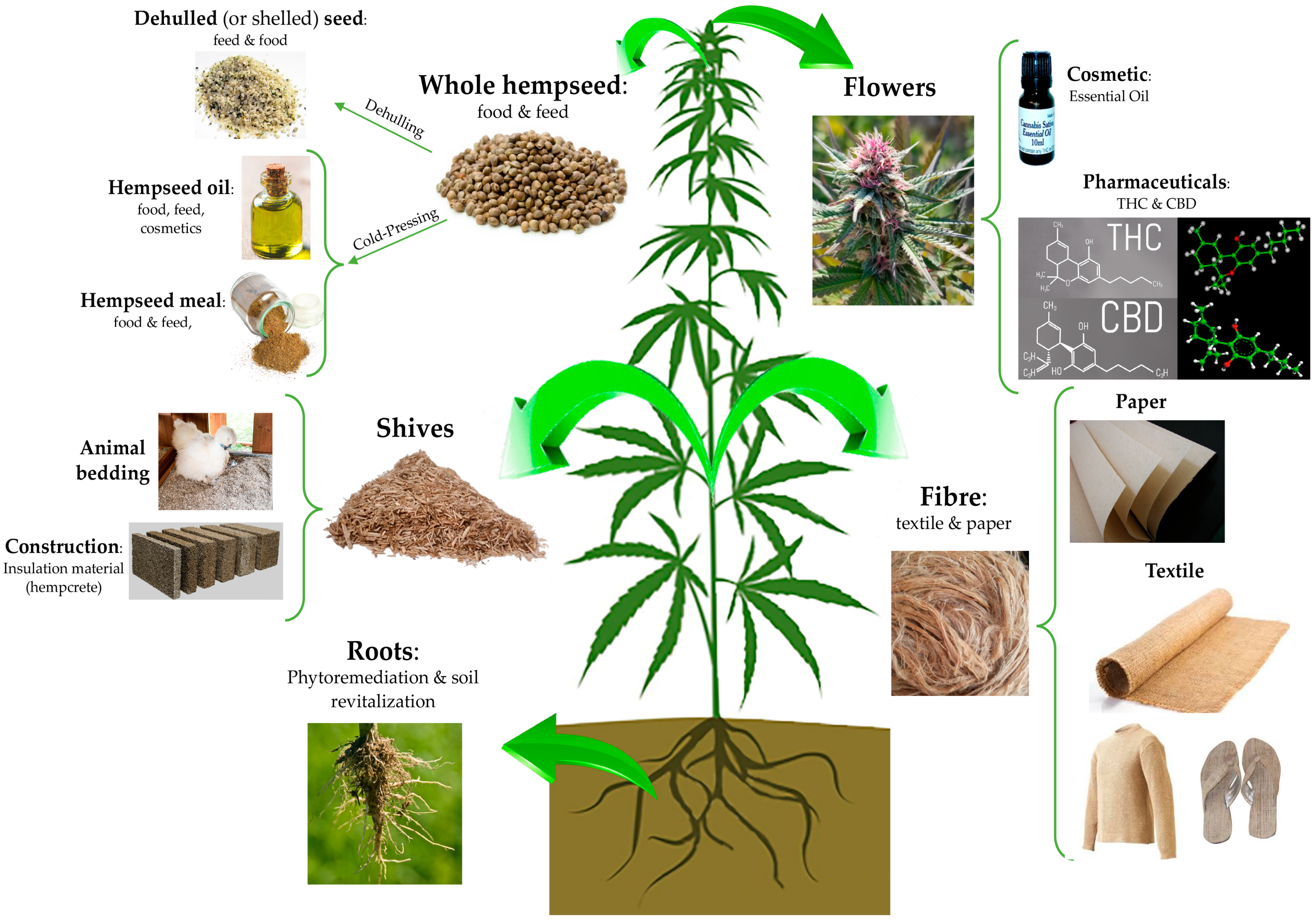
 “Acute Respiratory Distress Syndrome (ARDS) is a life-threatening complication that can ensue following Staphylococcus aureus infection. The enterotoxin produced by these bacteria (SEB) acts as a superantigen thereby activating a large proportion of T cells leading to cytokine storm and severe lung injury.
“Acute Respiratory Distress Syndrome (ARDS) is a life-threatening complication that can ensue following Staphylococcus aureus infection. The enterotoxin produced by these bacteria (SEB) acts as a superantigen thereby activating a large proportion of T cells leading to cytokine storm and severe lung injury. “Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis.
“Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis. “Extracts from the cannabis plant can dramatically improve the health of children suffering from refractory epilepsies such as Dravet syndrome.
“Extracts from the cannabis plant can dramatically improve the health of children suffering from refractory epilepsies such as Dravet syndrome. “Scientific research on how consumption of whole, natural Cannabis flower affects low mood and behavioral motivations more generally is largely nonexistent, and few studies to date have measured how common and commercially available Cannabis flower used in vivo may affect the experience of “depression” in real-time.
“Scientific research on how consumption of whole, natural Cannabis flower affects low mood and behavioral motivations more generally is largely nonexistent, and few studies to date have measured how common and commercially available Cannabis flower used in vivo may affect the experience of “depression” in real-time. “Studies have reported changes in the endocannabinoid system in the brain of patients with Alzheimer’s disease (AD), playing a role in the pathophysiology of AD. Cannabinoids have been shown to have neuroprotective properties, reduce neuroinflammation, and enhance neurogenesis. Evidence suggests that the utilization of marijuana products containing both tetrahydrocannabinol (THC) and cannabidiol (CBD) or CBD alone have been effective and safe for use in older people with agitation associated with dementia.
“Studies have reported changes in the endocannabinoid system in the brain of patients with Alzheimer’s disease (AD), playing a role in the pathophysiology of AD. Cannabinoids have been shown to have neuroprotective properties, reduce neuroinflammation, and enhance neurogenesis. Evidence suggests that the utilization of marijuana products containing both tetrahydrocannabinol (THC) and cannabidiol (CBD) or CBD alone have been effective and safe for use in older people with agitation associated with dementia. “Current antiepileptic drugs (AEDs) are undesirable for many reasons including the inability to reduce seizures in certain types of epilepsy, such as Dravet syndrome (DS) where in one-third of patients does not respond to current AEDs, and severe adverse effects that are frequently experienced by patients.
“Current antiepileptic drugs (AEDs) are undesirable for many reasons including the inability to reduce seizures in certain types of epilepsy, such as Dravet syndrome (DS) where in one-third of patients does not respond to current AEDs, and severe adverse effects that are frequently experienced by patients. “Attenuating emesis elicited by both disease and medical treatments of disease remains a critical
“Attenuating emesis elicited by both disease and medical treatments of disease remains a critical