 “Medicinal use of Cannabis sativa L. has an extensive history and it was essential in the discovery of phytocannabinoids, including the Cannabis major psychoactive compound-Δ9-tetrahydrocannabinol (Δ9-THC)-as well as the G-protein-coupled cannabinoid receptors (CBR), named cannabinoid receptor type-1 (CB1R) and cannabinoid receptor type-2 (CB2R), both part of the now known endocannabinoid system (ECS).
“Medicinal use of Cannabis sativa L. has an extensive history and it was essential in the discovery of phytocannabinoids, including the Cannabis major psychoactive compound-Δ9-tetrahydrocannabinol (Δ9-THC)-as well as the G-protein-coupled cannabinoid receptors (CBR), named cannabinoid receptor type-1 (CB1R) and cannabinoid receptor type-2 (CB2R), both part of the now known endocannabinoid system (ECS).
Cannabinoids is a vast term that defines several compounds that have been characterized in three categories: (i) endogenous, (ii) synthetic, and (iii) phytocannabinoids, and are able to modulate the CBR and ECS. Particularly, phytocannabinoids are natural terpenoids or phenolic compounds derived from Cannabis sativa.
However, these terpenoids and phenolic compounds can also be derived from other plants (non-cannabinoids) and still induce cannabinoid-like properties. Cannabimimetic ligands, beyond the Cannabis plant, can act as CBR agonists or antagonists, or ECS enzyme inhibitors, besides being able of playing a role in immune-mediated inflammatory and infectious diseases, neuroinflammatory, neurological, and neurodegenerative diseases, as well as in cancer, and autoimmunity by itself.
In this review, we summarize and critically highlight past, present, and future progress on the understanding of the role of cannabinoid-like molecules, mainly terpenes, as prospective therapeutics for different pathological conditions.”

 “Since antiquity, Cannabis has provoked enormous intrigue for its potential medicinal properties as well as for its unique pharmacological effects.
“Since antiquity, Cannabis has provoked enormous intrigue for its potential medicinal properties as well as for its unique pharmacological effects.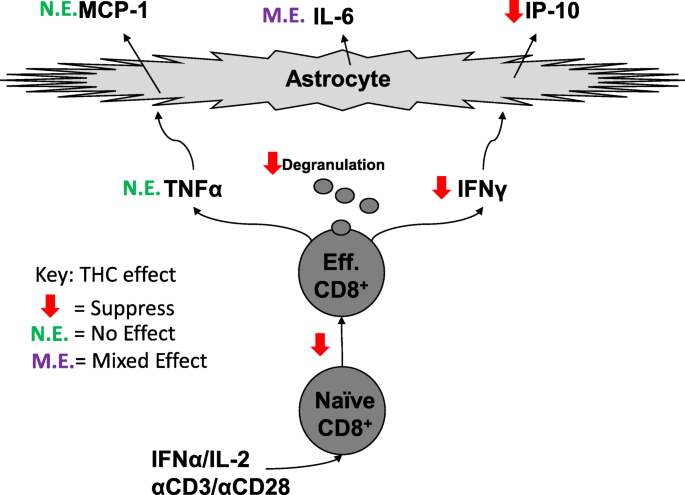 “CD8+ T cells can contribute to neuroinflammation by secretion of inflammatory cytokines like interferon γ (IFNγ) and tumor necrosis factor α (TNFα). Astrocytes, a glial cell in the brain, can be stimulated by IFNγ and TNFα to secrete the inflammatory cytokines, monocyte chemotactic protein 1 (MCP-1), interleukin 6 (IL-6), and interferon-γ inducible protein 10 (IP-10).
“CD8+ T cells can contribute to neuroinflammation by secretion of inflammatory cytokines like interferon γ (IFNγ) and tumor necrosis factor α (TNFα). Astrocytes, a glial cell in the brain, can be stimulated by IFNγ and TNFα to secrete the inflammatory cytokines, monocyte chemotactic protein 1 (MCP-1), interleukin 6 (IL-6), and interferon-γ inducible protein 10 (IP-10).
 “The present study investigated the effect of the lack of CB1 and CB2 receptors in mice ovarian morphology, folliculogenesis, oocyte retrieval, and oocyte maturation and evaluated the use of Δ9-tetrahydrocannabinol (THC) on oocyte in vitro maturation (IVM) by comparing classical IVM and two-step IVM by analyzing the meiotic competence of the oocytes and their evolution toward embryos.
“The present study investigated the effect of the lack of CB1 and CB2 receptors in mice ovarian morphology, folliculogenesis, oocyte retrieval, and oocyte maturation and evaluated the use of Δ9-tetrahydrocannabinol (THC) on oocyte in vitro maturation (IVM) by comparing classical IVM and two-step IVM by analyzing the meiotic competence of the oocytes and their evolution toward embryos. “Potential therapeutic actions of the cannabinoids delta-9-tetrahydrocannabinol (THC) and
“Potential therapeutic actions of the cannabinoids delta-9-tetrahydrocannabinol (THC) and  “To determine whether adjunctive dronabinol, a licensed form of delta-9-tetrahydrocannabinol, reduces opioid consumption when used off-label for managing acute pain following traumatic injury.
“To determine whether adjunctive dronabinol, a licensed form of delta-9-tetrahydrocannabinol, reduces opioid consumption when used off-label for managing acute pain following traumatic injury. “Excessive fear and anxiety, coupled with corticolimbic dysfunction, are core features of stress- and trauma-related psychopathology, such as posttraumatic stress disorder (PTSD).
“Excessive fear and anxiety, coupled with corticolimbic dysfunction, are core features of stress- and trauma-related psychopathology, such as posttraumatic stress disorder (PTSD).
 “Ratios of delta-9-tetrahydrocannabinol (THC) and
“Ratios of delta-9-tetrahydrocannabinol (THC) and