 “In traditional medicine, Cannabis sativa has been prescribed for a variety of diseases. Today, the plant is largely known for its recreational purpose, but it may find a way back to what it was originally known for: a herbal remedy. Most of the plant’s ingredients, such as Δ-tetrahydrocannabinol, cannabidiol, cannabigerol, and others, have demonstrated beneficial effects in preclinical models of intestinal inflammation. Endogenous cannabinoids (endocannabinoids) have shown a regulatory role in inflammation and mucosal permeability of the gastrointestinal tract where they likely interact with the gut microbiome. Anecdotal reports suggest that in humans, Cannabis exerts antinociceptive, anti-inflammatory, and antidiarrheal properties. Despite these reports, strong evidence on beneficial effects of Cannabis in human gastrointestinal diseases is lacking. Clinical trials with Cannabis in patients suffering from inflammatory bowel disease (IBD) have shown improvement in quality of life but failed to provide evidence for a reduction of inflammation markers. Within the endogenous opioid system, mu opioid receptors may be involved in anti-inflammation of the gut. Opioids are frequently used to treat abdominal pain in IBD; however, heavy opioid use in IBD is associated with opioid dependency and higher mortality. This review highlights latest advances in the potential treatment of IBD using Cannabis/cannabinoids or opioids.”
“In traditional medicine, Cannabis sativa has been prescribed for a variety of diseases. Today, the plant is largely known for its recreational purpose, but it may find a way back to what it was originally known for: a herbal remedy. Most of the plant’s ingredients, such as Δ-tetrahydrocannabinol, cannabidiol, cannabigerol, and others, have demonstrated beneficial effects in preclinical models of intestinal inflammation. Endogenous cannabinoids (endocannabinoids) have shown a regulatory role in inflammation and mucosal permeability of the gastrointestinal tract where they likely interact with the gut microbiome. Anecdotal reports suggest that in humans, Cannabis exerts antinociceptive, anti-inflammatory, and antidiarrheal properties. Despite these reports, strong evidence on beneficial effects of Cannabis in human gastrointestinal diseases is lacking. Clinical trials with Cannabis in patients suffering from inflammatory bowel disease (IBD) have shown improvement in quality of life but failed to provide evidence for a reduction of inflammation markers. Within the endogenous opioid system, mu opioid receptors may be involved in anti-inflammation of the gut. Opioids are frequently used to treat abdominal pain in IBD; however, heavy opioid use in IBD is associated with opioid dependency and higher mortality. This review highlights latest advances in the potential treatment of IBD using Cannabis/cannabinoids or opioids.”
https://www.ncbi.nlm.nih.gov/pubmed/31899693
https://journals.lww.com/ctg/Abstract/latest/Cannabinoids_and_Opioids_in_the_Treatment_of.99898.aspx

 “Cannabinoids are pharmacologically active agents extracted from the cannabis plant.
“Cannabinoids are pharmacologically active agents extracted from the cannabis plant. 
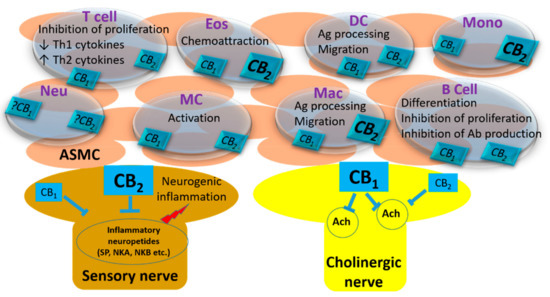
 “Even a brief exposure to severe stress strengthens synaptic connectivity days later in the amygdala, a brain area implicated in the affective symptoms of stress-related psychiatric disorders. However, little is known about the synaptic signaling mechanisms during stress that eventually culminate in its delayed impact on the amygdala. Hence, we investigated early stress-induced changes in amygdalar synaptic signaling in order to prevent its delayed effects.
“Even a brief exposure to severe stress strengthens synaptic connectivity days later in the amygdala, a brain area implicated in the affective symptoms of stress-related psychiatric disorders. However, little is known about the synaptic signaling mechanisms during stress that eventually culminate in its delayed impact on the amygdala. Hence, we investigated early stress-induced changes in amygdalar synaptic signaling in order to prevent its delayed effects. “The most bioactive ingredient of
“The most bioactive ingredient of  “The endocannabinoid system (ECS), modulated by metabolites of linoleic acid (LA), is important in regulating cardiovascular function.
“The endocannabinoid system (ECS), modulated by metabolites of linoleic acid (LA), is important in regulating cardiovascular function.
 “Excessive binge alcohol drinking may adversely affect cardiovascular function. In this study we characterize the detailed hemodynamic effects of an acute alcohol binge in mice using multiple approaches and investigate the role of the endocannabinoid-
“Excessive binge alcohol drinking may adversely affect cardiovascular function. In this study we characterize the detailed hemodynamic effects of an acute alcohol binge in mice using multiple approaches and investigate the role of the endocannabinoid-