 “This review is to summarize most recent evidence published in the last 18 months on medical and recreational use of cannabis and cannabinoids in relation to anxiety, depression (unipolar and bipolar), and dysregulation of emotions as part of posttraumatic stress disorders (PTSD) and emotionally instable personality disorders.
“This review is to summarize most recent evidence published in the last 18 months on medical and recreational use of cannabis and cannabinoids in relation to anxiety, depression (unipolar and bipolar), and dysregulation of emotions as part of posttraumatic stress disorders (PTSD) and emotionally instable personality disorders.
It also covers the investigation of endocannabinoids as potential biomarkers in these conditions. This is important with increasing medicinal use of cannabinoids and growing social tolerance towards recreational cannabis use.
RECENT FINDINGS:
There is some recent evidence suggesting cannabinoids, cannabidiol or cannabidiol-enriched cannabis preparations have anxiolytic properties. In addition, depression may be worsened by cannabis use, however, randomized controlled trials (RCT) are lacking.
New evidence also suggests that cannabidiol or cannabidiol-enriched cannabis use for PTSD and emotion regulation can induce hyporesponse to fear and stress. Further, several lines of evidence point to the endocannabinoid system as a key player in some of the reviewed disorders, in particular anxiety and PTSD.
SUMMARY:
The most recent evidence for a therapeutic use of cannabinoids in the reviewed conditions is weak and lacking well designed RCTs. However, there is some indication of the role of the endocannabinoid system in these conditions that warrant further studies.”
https://www.ncbi.nlm.nih.gov/pubmed/31714262
https://insights.ovid.com/crossref?an=00001504-900000000-99165

 “Endocannabinoids are produced within the gastrointestinal (GI) tract and modulate energy homeostasis and food intake, at least in part, via vagally-dependent actions. The recent paper by Christie et al., [Christie, et al. J Physiol, 2019] demonstrate, for the first time, that
“Endocannabinoids are produced within the gastrointestinal (GI) tract and modulate energy homeostasis and food intake, at least in part, via vagally-dependent actions. The recent paper by Christie et al., [Christie, et al. J Physiol, 2019] demonstrate, for the first time, that  “Brain trauma was clinically associated with increased osteogenesis in the appendicular skeleton. We showed previously in C57BL/6J mice that mild traumatic brain injury (mTBI) transiently induced bone formation in the femur via the
“Brain trauma was clinically associated with increased osteogenesis in the appendicular skeleton. We showed previously in C57BL/6J mice that mild traumatic brain injury (mTBI) transiently induced bone formation in the femur via the  “The endocannabinoid (eCB) system, i.e. the receptors that respond to the psychoactive component of
“The endocannabinoid (eCB) system, i.e. the receptors that respond to the psychoactive component of  “The anticancer effects of the omega-3 long chain polyunsaturated fatty acids (LCPUFA), EPA and DHA may be due, at least in part, to conversion to their respective endocannabinoid derivatives, eicosapentaenoyl-ethanolamine (EPEA) and docosahexaenoyl-ethanolamine (DHEA).
“The anticancer effects of the omega-3 long chain polyunsaturated fatty acids (LCPUFA), EPA and DHA may be due, at least in part, to conversion to their respective endocannabinoid derivatives, eicosapentaenoyl-ethanolamine (EPEA) and docosahexaenoyl-ethanolamine (DHEA). “The role of the endocannabinoid system in stress-related psychiatric symptoms has been investigated in many animal and human studies.
“The role of the endocannabinoid system in stress-related psychiatric symptoms has been investigated in many animal and human studies. “Hepatic fibrosis is the consequence of an unresolved wound healing process in response to chronic liver injury and involves multiple cell types and molecular mechanisms. The hepatic endocannabinoid and apelin systems are two signalling pathways with a substantial role in the liver fibrosis pathophysiology-both are upregulated in patients with advanced liver disease. Endogenous
“Hepatic fibrosis is the consequence of an unresolved wound healing process in response to chronic liver injury and involves multiple cell types and molecular mechanisms. The hepatic endocannabinoid and apelin systems are two signalling pathways with a substantial role in the liver fibrosis pathophysiology-both are upregulated in patients with advanced liver disease. Endogenous 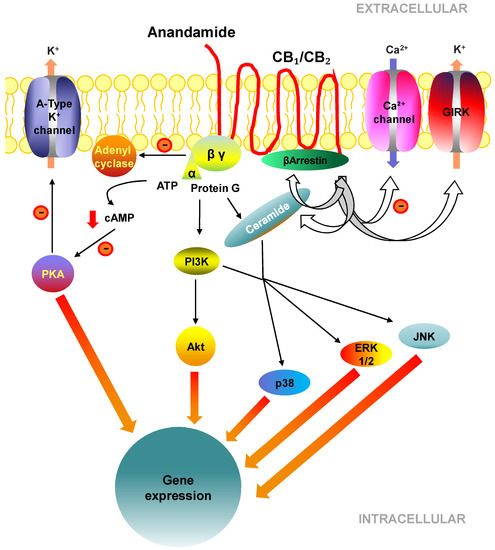
 “Memory and GABAergic activity in the hippocampus of stressed rats improve after n-3 polyunsaturated fatty acid (PUFA) supplementation.
“Memory and GABAergic activity in the hippocampus of stressed rats improve after n-3 polyunsaturated fatty acid (PUFA) supplementation. “Treating Cancer-induced bone pain (CIBP) continues to be a major clinical challenge and underlying mechanisms of CIBP remain unclear.
“Treating Cancer-induced bone pain (CIBP) continues to be a major clinical challenge and underlying mechanisms of CIBP remain unclear. “Microglia are the resident, innate immune cells of the central nervous system (CNS) and are critical in managing CNS injuries and infections. Microglia also maintain CNS homeostasis by influencing neuronal development, viability, and function. However, aberrant microglial activity and phenotypes are associated with CNS pathology, including autism spectrum disorder (ASD). Thus, improving our knowledge of microglial regulation could provide insights into the maintenance of CNS homeostasis as well as the prevention and treatment of ASD.
“Microglia are the resident, innate immune cells of the central nervous system (CNS) and are critical in managing CNS injuries and infections. Microglia also maintain CNS homeostasis by influencing neuronal development, viability, and function. However, aberrant microglial activity and phenotypes are associated with CNS pathology, including autism spectrum disorder (ASD). Thus, improving our knowledge of microglial regulation could provide insights into the maintenance of CNS homeostasis as well as the prevention and treatment of ASD.