 “Anxiety disorders have the highest lifetime prevalence of any mental illness worldwide, leading to high societal costs and economic burden. Current pharmacotherapies for anxiety disorders are associated with adverse effects and low efficacy.
“Anxiety disorders have the highest lifetime prevalence of any mental illness worldwide, leading to high societal costs and economic burden. Current pharmacotherapies for anxiety disorders are associated with adverse effects and low efficacy.
Cannabidiol (CBD) is a constituent of the Cannabis plant, which has potential therapeutic properties for various indications. After the recent legalization of cannabis, CBD has drawn increased attention as a potential treatment, as the majority of existing data suggest it is safe, well tolerated, has few adverse effects, and demonstrates no potential for abuse or dependence in humans.
Pre-clinical research using animal models of innate fear and anxiety-like behaviors have found anxiolytic, antistress, anticompulsive, and panicolytic-like effects of CBD. Preliminary evidence from human trials using both healthy volunteers and individuals with social anxiety disorder, suggests that CBD may have anxiolytic effects.
Although these findings are promising, future research is warranted to determine the efficacy of CBD in other anxiety disorders, establish appropriate doses, and determine its long-term efficacy. The majority of pre-clinical and clinical research has been conducted using males only. Among individuals with anxiety disorders, the prevalence rates, symptomology, and treatment response differ between males and females. Thus, future research should focus on this area due to the lack of research in females and the knowledge gap on sex and gender differences in the effectiveness of CBD as a potential treatment for anxiety.”
https://pubmed.ncbi.nlm.nih.gov/32923656/
“Cannabidiol (CBD) is a constituent of the Cannabis plant, which has potential therapeutic properties across many neuropsychiatric disorders. Overall, existing pre-clinical and clinical evidence supports a possible role for CBD as a novel treatment for anxiety disorders.”

 “Emotional dysregulation and anxiety are common in people at clinical high risk for psychosis (CHR) and are associated with altered neural responses to emotional stimuli in the striatum and medial temporal lobe.
“Emotional dysregulation and anxiety are common in people at clinical high risk for psychosis (CHR) and are associated with altered neural responses to emotional stimuli in the striatum and medial temporal lobe. “UVB phototherapy is treatment for psoriasis, which increases phospholipid oxidative modifications in the cell membrane of the skin. Therefore, we carried out lipidomic analysis on the keratinocytes of healthy individuals and patients with psoriasis irradiated with UVB and treated with cannabidiol (CBD), phytocannabinoid with antioxidant and anti-inflammatory properties.
“UVB phototherapy is treatment for psoriasis, which increases phospholipid oxidative modifications in the cell membrane of the skin. Therefore, we carried out lipidomic analysis on the keratinocytes of healthy individuals and patients with psoriasis irradiated with UVB and treated with cannabidiol (CBD), phytocannabinoid with antioxidant and anti-inflammatory properties.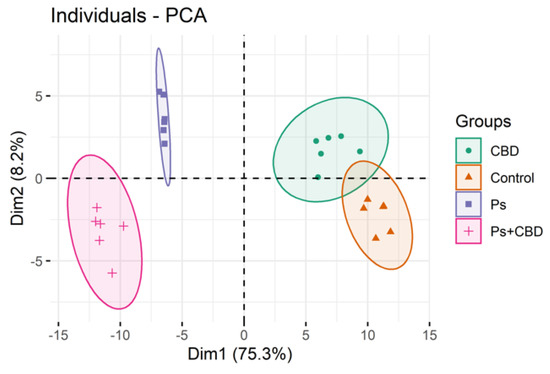
 “Highly purified cannabidiol (CBD) has demonstrated efficacy with an acceptable safety profile in patients with Lennox-Gastaut syndrome or Dravet syndrome in randomized, double-blind, add-on, controlled phase 3 trials.
“Highly purified cannabidiol (CBD) has demonstrated efficacy with an acceptable safety profile in patients with Lennox-Gastaut syndrome or Dravet syndrome in randomized, double-blind, add-on, controlled phase 3 trials. “Mutations in SYNGAP1 are associated with developmental delay, epilepsy, and autism spectrum disorder (ASD). Epilepsy is often drug-resistant in this syndrome with frequent drop attacks.
“Mutations in SYNGAP1 are associated with developmental delay, epilepsy, and autism spectrum disorder (ASD). Epilepsy is often drug-resistant in this syndrome with frequent drop attacks. “In the present study, the antimicrobial effect of Cannabis sativa Futura 75 was evaluated both in vitro against foodborne bacterial pathogens, and on food against naturally occurring microbial groups of minced meat stored for 8 days at 4°C.
“In the present study, the antimicrobial effect of Cannabis sativa Futura 75 was evaluated both in vitro against foodborne bacterial pathogens, and on food against naturally occurring microbial groups of minced meat stored for 8 days at 4°C. “Hemp (Cannabis sativa L.) seed contains high contents of various nutrients, including fatty acids and proteins.
“Hemp (Cannabis sativa L.) seed contains high contents of various nutrients, including fatty acids and proteins.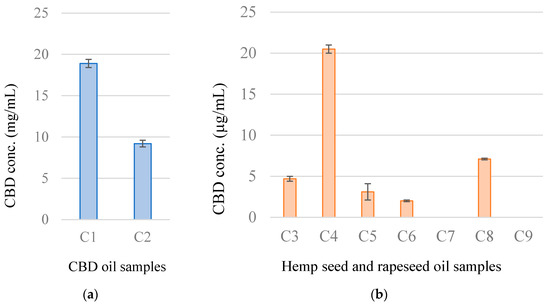
 “Industrial hemp (Cannabis sativa L., Cannabaceae) is an ancient cultivated plant originating from Central Asia and historically has been a multi-use crop valued for its fiber, food, and medicinal uses. Various oriental and Asian cultures kept records of its production and numerous uses.
“Industrial hemp (Cannabis sativa L., Cannabaceae) is an ancient cultivated plant originating from Central Asia and historically has been a multi-use crop valued for its fiber, food, and medicinal uses. Various oriental and Asian cultures kept records of its production and numerous uses.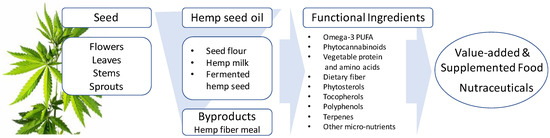
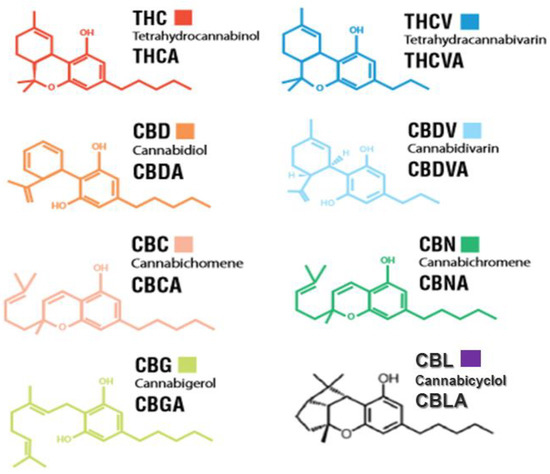
 “The immune-suppressive effects of cannabidiol (CBD) are attributed to the modulation of essential immunological signaling pathways and receptors. Mechanistic understanding of the pharmacological effects of CBD emphasizes the therapeutic potential of CBD as a novel immune modulator.
“The immune-suppressive effects of cannabidiol (CBD) are attributed to the modulation of essential immunological signaling pathways and receptors. Mechanistic understanding of the pharmacological effects of CBD emphasizes the therapeutic potential of CBD as a novel immune modulator.