 “Cannabis sativa L. is an ancient medicinal plant wherefrom over 120 cannabinoids are extracted. In the past two decades, there has been increasing interest in the therapeutic potential of cannabis-based treatments for neurological disorders such as epilepsy, and there is now evidence for the medical use of cannabis and its effectiveness for a wide range of diseases.
“Cannabis sativa L. is an ancient medicinal plant wherefrom over 120 cannabinoids are extracted. In the past two decades, there has been increasing interest in the therapeutic potential of cannabis-based treatments for neurological disorders such as epilepsy, and there is now evidence for the medical use of cannabis and its effectiveness for a wide range of diseases.
Cannabinoid treatments for pain and spasticity in patients with multiple sclerosis (Nabiximols) have been approved in several countries. Cannabidiol (CBD), in contrast to tetra-hydro-cannabidiol (THC), is not a controlled substance in the European Union, and over the years there has been increasing use of CBD-enriched extracts and pure CBD for seizure disorders, particularly in children. No analytical controls are mandatory for CBD-based products and a pronounced variability in CBD concentrations in commercialized CBD oil preparations has been identified.
Randomized controlled trials of plant-derived CBD for treatment of Lennox-Gastaut syndrome (LGS) and Dravet syndrome (DS) have provided evidence of anti-seizure effects, and in June 2018, CBD was approved by the Food and Drug Administration as an add-on antiepileptic drug for patients two years of age and older with LGS or DS. Medical cannabis, with various ratios of CBD and THC and in different galenic preparations, is licensed in many European countries for several indications, and in July 2019, the European Medicines Agency also granted marketing authorisation for CBD in association with clobazam, for the treatment of seizures associated with LGS or DS.
The purpose of this article is to review the availability of cannabis-based products and cannabinoid-based medicines, together with current regulations regarding indications in Europe (as of July 2019). The lack of approval by the central agencies, as well as social and political influences, have led to significant variation in usage between countries.”
https://www.ncbi.nlm.nih.gov/pubmed/31941643
https://www.jle.com/fr/revues/epd/e-docs/source_of_cannabinoids_what_is_available_what_is_used_and_where_does_it_come_from__316043/article.phtml
 “The legalization of cannabis in some states has intensified interest in the potential for cannabis and its constituents to lead to novel therapeutics for pain.
“The legalization of cannabis in some states has intensified interest in the potential for cannabis and its constituents to lead to novel therapeutics for pain.
 “Endo-, phyto- and synthetic cannabinoids have been proposed as promising anti-cancer agents able to impair cancer cells’ behavior without affecting their non-transformed counterparts.
“Endo-, phyto- and synthetic cannabinoids have been proposed as promising anti-cancer agents able to impair cancer cells’ behavior without affecting their non-transformed counterparts.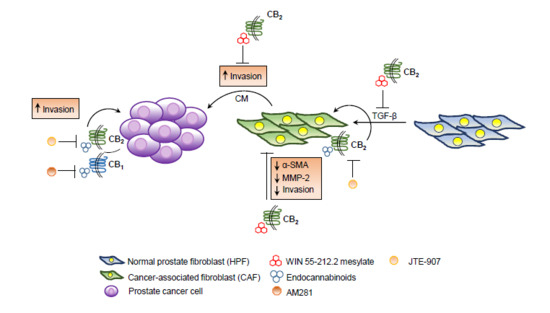
 “Recent evidence suggests that
“Recent evidence suggests that  “Palliative medicine physicians are challenged by lack of guidance regarding effectiveness and dosing of cannabis products in the setting of their emerging popularity.
“Palliative medicine physicians are challenged by lack of guidance regarding effectiveness and dosing of cannabis products in the setting of their emerging popularity. “Given the growing challenges in chronic pain management coupled with the ongoing consequences of the opioid epidemic, pain management practitioners are looking into more effective, innovative, and safer alternatives to treat pain.
“Given the growing challenges in chronic pain management coupled with the ongoing consequences of the opioid epidemic, pain management practitioners are looking into more effective, innovative, and safer alternatives to treat pain. “Abnormal
“Abnormal  “Cannabis sativa L. is an ancient medicinal plant wherefrom over 120 cannabinoids are extracted. In the past two decades, there has been increasing interest in the therapeutic potential of cannabis-based treatments for neurological disorders such as epilepsy, and there is now evidence for the medical use of cannabis and its effectiveness for a wide range of diseases.
“Cannabis sativa L. is an ancient medicinal plant wherefrom over 120 cannabinoids are extracted. In the past two decades, there has been increasing interest in the therapeutic potential of cannabis-based treatments for neurological disorders such as epilepsy, and there is now evidence for the medical use of cannabis and its effectiveness for a wide range of diseases.
 “Neurofibromatosis type 1 (NF1) is a common genetic disorder. Pain is a major symptom of this disease which can be secondary to the development of plexiform and subcutaneous neurofibromas, musculoskeletal symptoms (such as scoliosis and pseudoarthrosis), and headaches. Visible neurofibromas add significant psychosocial distress for NF1 patients. Along with the chronic pain, psychosocial distress contributes to associated mood disorders, such as depression and anxiety.
“Neurofibromatosis type 1 (NF1) is a common genetic disorder. Pain is a major symptom of this disease which can be secondary to the development of plexiform and subcutaneous neurofibromas, musculoskeletal symptoms (such as scoliosis and pseudoarthrosis), and headaches. Visible neurofibromas add significant psychosocial distress for NF1 patients. Along with the chronic pain, psychosocial distress contributes to associated mood disorders, such as depression and anxiety.