 “Importance: Frontline health care professionals who work with patients with COVID-19 have an increased incidence of burnout symptoms. Cannabidiol (CBD) has anxiolytic and antidepressant properties and may be capable of reducing emotional exhaustion and burnout symptoms.
“Importance: Frontline health care professionals who work with patients with COVID-19 have an increased incidence of burnout symptoms. Cannabidiol (CBD) has anxiolytic and antidepressant properties and may be capable of reducing emotional exhaustion and burnout symptoms.
Objective: To investigate the safety and efficacy of CBD therapy for the reduction of emotional exhaustion and burnout symptoms among frontline health care professionals working with patients with COVID-19.
Interventions: Cannabidiol, 300 mg (150 mg twice per day), plus standard care or standard care alone for 28 days.
Main outcomes and measures: The primary outcome was emotional exhaustion and burnout symptoms, which were assessed for 28 days using the emotional exhaustion subscale of the Brazilian version of the Maslach Burnout Inventory-Human Services Survey for Medical Personnel.
Results: A total of 120 participants were randomized to receive either CBD, 300 mg, plus standard care (treatment arm; n = 61) or standard care alone (control arm; n = 59) for 28 days. Of those, 118 participants (59 participants in each arm; 79 women [66.9%]; mean age, 33.6 years [95% CI, 32.3-34.9 years]) received the intervention and were included in the efficacy analysis. In the treatment arm, scores on the emotional exhaustion subscale of the Maslach Burnout Inventory significantly decreased at day 14 (mean difference, 4.14 points; 95% CI, 1.47-6.80 points; partial eta squared [ηp2] = 0.08), day 21 (mean difference, 4.34 points; 95% CI, 0.94-7.73 points; ηp2 = 0.05), and day 28 (mean difference, 4.01 points; 95% CI, 0.43-7.59 points; ηp2 = 0.04). However, 5 participants, all of whom were in the treatment group, experienced serious adverse events: 4 cases of elevated liver enzymes (1 critical and 3 mild, with the mild elevations reported at the final 28-day assessment) and 1 case of severe pharmacodermia. In 2 of those cases (1 with critical elevation of liver enzymes and 1 with severe pharmacodermia), CBD therapy was discontinued, and the participants had a full recovery.
Conclusions and relevance: In this study, CBD therapy reduced symptoms of burnout and emotional exhaustion among health care professionals working with patients during the COVID-19 pandemic. However, it is necessary to balance the benefits of CBD therapy with potential undesired or adverse effects. Future double-blind placebo-controlled clinical trials are needed to confirm the present findings.”
https://pubmed.ncbi.nlm.nih.gov/34387679/
“Daily administration of CBD, 300 mg, combined with standard care reduced the symptoms and diagnoses of anxiety, depression, and emotional exhaustion among frontline health care professionals working with patients with COVID-19. Cannabidiol may act as an effective agent for the reduction of burnout symptoms among a population with important mental health needs worldwide.”
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2782994
“Mitochondria have the main roles in myocardial tissue homeostasis, through providing ATP for the vital enzymes in intermediate metabolism, contractile apparatus and maintaining ion homeostasis. Mitochondria-related cardiotoxicity results from the exposure with illicit drugs have previously reported. These illicit drugs interference with processes of normal mitochondrial homeostasis and lead to mitochondrial dysfunction and mitochondrial-related oxidative stress.

 “Colic is a common digestive disorder in horses and one of the most urgent problems in equine medicine. A growing body of literature has indicated that the activation of cannabinoid receptors could exert beneficial effects on gastrointestinal inflammation and visceral hypersensitivity.
“Colic is a common digestive disorder in horses and one of the most urgent problems in equine medicine. A growing body of literature has indicated that the activation of cannabinoid receptors could exert beneficial effects on gastrointestinal inflammation and visceral hypersensitivity.  “Cannabidiol is increasingly considered for treatment of a wide range of medical conditions.
“Cannabidiol is increasingly considered for treatment of a wide range of medical conditions.  “Background and purpose:
“Background and purpose: 
 “Importance:
“Importance:  “Cannabis sativa
“Cannabis sativa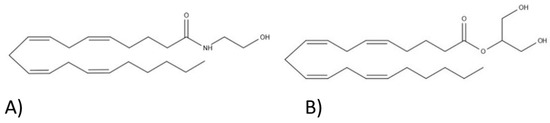
 “The Cannabis sativa plant has been used medicinally and recreationally for thousands of years, but recently only relatively some of its constituents have been identified.
“The Cannabis sativa plant has been used medicinally and recreationally for thousands of years, but recently only relatively some of its constituents have been identified. 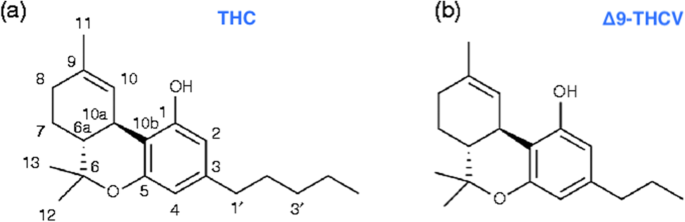 “Δ9-Tetrahydrocannabivarin (THCV) is a cannabis-derived compound with unique properties that set it apart from the more common cannabinoids, such as Δ9-tetrahydrocannabinol (THC). The main advantage of THCV over THC is the lack of psychoactive effects.
“Δ9-Tetrahydrocannabivarin (THCV) is a cannabis-derived compound with unique properties that set it apart from the more common cannabinoids, such as Δ9-tetrahydrocannabinol (THC). The main advantage of THCV over THC is the lack of psychoactive effects.