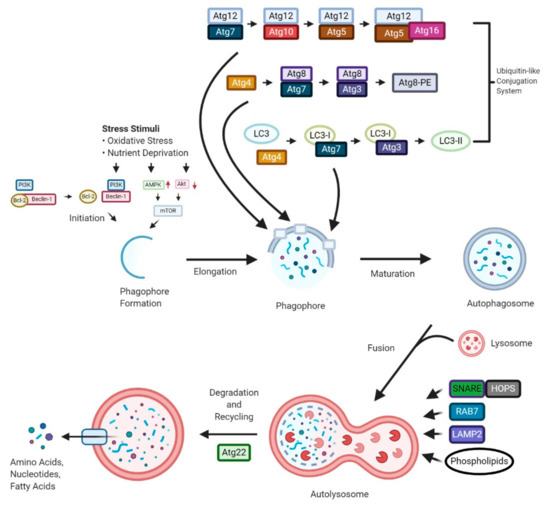
“Autophagy is a “self-degradation” process whereby malfunctioned cytoplasmic constituents and protein aggregates are engulfed by a vesicle called the autophagosome, and subsequently degraded by the lysosome. Autophagy plays a crucial role in sustaining protein homeostasis and can be an alternative source of energy under detrimental circumstances. Studies have demonstrated a paradoxical function for autophagy in cancer, displaying both tumour suppressive and tumour promotive roles. In early phases of tumour development autophagy promotes cancer cell death. In later phases, autophagy enables cancer cells to survive and withstand therapy.
“Autophagy is a “self-degradation” process whereby malfunctioned cytoplasmic constituents and protein aggregates are engulfed by a vesicle called the autophagosome, and subsequently degraded by the lysosome. Autophagy plays a crucial role in sustaining protein homeostasis and can be an alternative source of energy under detrimental circumstances. Studies have demonstrated a paradoxical function for autophagy in cancer, displaying both tumour suppressive and tumour promotive roles. In early phases of tumour development autophagy promotes cancer cell death. In later phases, autophagy enables cancer cells to survive and withstand therapy.
Cannabinoids, which are derivatives of the Cannabis sativa L. plant, have shown to be associated with autophagy induction in cells. There is an emerging interest in studying the signalling pathways involved in cannabinoid-induced autophagy and their potential application in anticancer therapies. In this review, the molecular mechanisms involved in the autophagy degradation process will be discussed. This review also highlights a role for autophagy in cancer progression, with cannabinoid-induced autophagy presenting a novel strategy for anticancer therapy.”
https://pubmed.ncbi.nlm.nih.gov/33802014/


 “Purpose: Hodgkin lymphoma (HL) is the fourth most frequent cancer diagnosis among pregnant females. A multidisciplinary team is mandatory to obtain the best treatment and prognosis for the mother and for the baby. Here, we present the case of a patient diagnosed with HL and its evolution during 2 pregnancies.
“Purpose: Hodgkin lymphoma (HL) is the fourth most frequent cancer diagnosis among pregnant females. A multidisciplinary team is mandatory to obtain the best treatment and prognosis for the mother and for the baby. Here, we present the case of a patient diagnosed with HL and its evolution during 2 pregnancies. “The cannabis plant (Cannabis sativa L.) produces an estimated 545 chemical compounds of different biogenetic classes. In addition to economic value, many of these phytochemicals have medicinal and physiological activity. The plant is most popularly known for its two most-prominent and most-studied secondary metabolites-Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD). Both Δ9-THC and CBD have a wide therapeutic window across many ailments and form part of a class of secondary metabolites called cannabinoids-of which approximately over 104 exist.
“The cannabis plant (Cannabis sativa L.) produces an estimated 545 chemical compounds of different biogenetic classes. In addition to economic value, many of these phytochemicals have medicinal and physiological activity. The plant is most popularly known for its two most-prominent and most-studied secondary metabolites-Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD). Both Δ9-THC and CBD have a wide therapeutic window across many ailments and form part of a class of secondary metabolites called cannabinoids-of which approximately over 104 exist. “Breast cancer is the leading cause of cancer-related death in women worldwide. In the last years, cannabinoids have gained attention in the clinical setting and clinical trials with cannabinoid-based preparations are underway. However, contradictory anti-tumour properties have also been reported. Thus, the elucidation of the molecular mechanisms behind their anti-tumour efficacy is crucial to better understand its therapeutic potential.
“Breast cancer is the leading cause of cancer-related death in women worldwide. In the last years, cannabinoids have gained attention in the clinical setting and clinical trials with cannabinoid-based preparations are underway. However, contradictory anti-tumour properties have also been reported. Thus, the elucidation of the molecular mechanisms behind their anti-tumour efficacy is crucial to better understand its therapeutic potential. “Cannabis was extensively utilized for its medicinal properties till the 19th century. A steep decline in its medicinal usage was observed later due to its emergence as an illegal recreational drug. Advances in technology and scientific findings led to the discovery of delta-9-tetrahydrocannabinol (THC), the primary psychoactive compound of cannabis, that further led to the discovery of endogenous cannabinoids system consisting of G-protein-coupled receptors – cannabinoid receptor 1 and cannabinoid receptor 2 along with their ligands, mainly anandamide and 2-arachidonoylglycerol. Endocannabinoid (EC) is shown to be a modulator not only for physiological functions but also for the immune system, endocrine network, and central nervous system. Medicinal research and meta-data analysis over the last few decades have shown a significant potential for both THC and cannabidiol (CBD) to exert palliative effects. People suffering from many forms of advanced stages of cancers undergo chemotherapy-induced nausea and vomiting followed by severe and chronic neuropathic pain and weight loss. THC and CBD exhibit effective analgesic, anxiolytic, and appetite-stimulating effect on patients suffering from cancer. Drugs currently available in the market to treat such chemotherapy-induced cancer-related ailments are Sativex (GW Pharmaceutical), Dronabinol (Unimed Pharmaceuticals), and Nabilone (Valeant Pharmaceuticals). Apart from exerting palliative effects, THC also shows promising role in the treatment of cancer growth, neurodegenerative diseases (multiple sclerosis and Alzheimer’s disease), and alcohol addiction and hence should be exploited for potential benefits. The current review discusses the nature and role of CB receptors, specific applications of cannabinoids, and major studies that have assessed the role of cannabinoids in cancer management.”
“Cannabis was extensively utilized for its medicinal properties till the 19th century. A steep decline in its medicinal usage was observed later due to its emergence as an illegal recreational drug. Advances in technology and scientific findings led to the discovery of delta-9-tetrahydrocannabinol (THC), the primary psychoactive compound of cannabis, that further led to the discovery of endogenous cannabinoids system consisting of G-protein-coupled receptors – cannabinoid receptor 1 and cannabinoid receptor 2 along with their ligands, mainly anandamide and 2-arachidonoylglycerol. Endocannabinoid (EC) is shown to be a modulator not only for physiological functions but also for the immune system, endocrine network, and central nervous system. Medicinal research and meta-data analysis over the last few decades have shown a significant potential for both THC and cannabidiol (CBD) to exert palliative effects. People suffering from many forms of advanced stages of cancers undergo chemotherapy-induced nausea and vomiting followed by severe and chronic neuropathic pain and weight loss. THC and CBD exhibit effective analgesic, anxiolytic, and appetite-stimulating effect on patients suffering from cancer. Drugs currently available in the market to treat such chemotherapy-induced cancer-related ailments are Sativex (GW Pharmaceutical), Dronabinol (Unimed Pharmaceuticals), and Nabilone (Valeant Pharmaceuticals). Apart from exerting palliative effects, THC also shows promising role in the treatment of cancer growth, neurodegenerative diseases (multiple sclerosis and Alzheimer’s disease), and alcohol addiction and hence should be exploited for potential benefits. The current review discusses the nature and role of CB receptors, specific applications of cannabinoids, and major studies that have assessed the role of cannabinoids in cancer management.” “The main aspects of severe COVID-19 disease pathogenesis include hyper-induction of proinflammatory cytokines, also known as ‘cytokine storm’, that precedes acute respiratory distress syndrome (ARDS) and often leads to death. COVID-19 patients often suffer from lung fibrosis, a serious and untreatable condition. There remains no effective treatment for these complications.
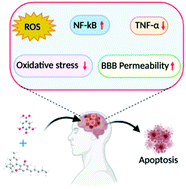
“The main aspects of severe COVID-19 disease pathogenesis include hyper-induction of proinflammatory cytokines, also known as ‘cytokine storm’, that precedes acute respiratory distress syndrome (ARDS) and often leads to death. COVID-19 patients often suffer from lung fibrosis, a serious and untreatable condition. There remains no effective treatment for these complications. “Inflammasomes are cytoplasmic inflammatory signaling protein complexes that detect microbial materials, sterile inflammatory insults, and certain host-derived elements. Inflammasomes, once activated, promote caspase-1-mediated maturation and secretion of pro-inflammatory cytokines, interleukin (IL)-1β and IL-18, leading to pyroptosis. Current advances in inflammasome research support their involvement in the development of chronic inflammatory disorders in contrast to their role in regulating innate immunity.
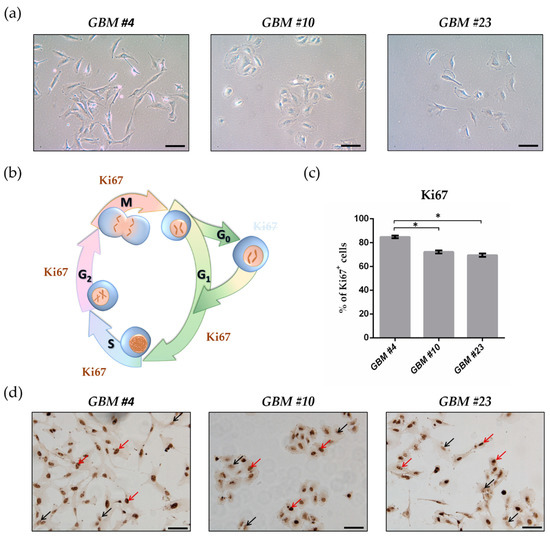
“Inflammasomes are cytoplasmic inflammatory signaling protein complexes that detect microbial materials, sterile inflammatory insults, and certain host-derived elements. Inflammasomes, once activated, promote caspase-1-mediated maturation and secretion of pro-inflammatory cytokines, interleukin (IL)-1β and IL-18, leading to pyroptosis. Current advances in inflammasome research support their involvement in the development of chronic inflammatory disorders in contrast to their role in regulating innate immunity. “Cannabidiol (CBD) has been shown to slow cancer cell growth and is toxic to human glioblastoma cell lines. Thus, CBD could be an effective therapeutic for glioblastoma.
“Cannabidiol (CBD) has been shown to slow cancer cell growth and is toxic to human glioblastoma cell lines. Thus, CBD could be an effective therapeutic for glioblastoma.