 “The market for products featuring hemp extracts is large and growing larger. However, safety concerns have been raised by medical and regulatory agencies. Post marketing surveillance of full spectrum hemp extract (FSHE) products manufactured and distributed by CV Sciences (CVSI) and traded under the brand PlusCBD™ was conducted over a 2-year period (2018-2019). The safety of these products was assessed by analyzing adverse events reports.
“The market for products featuring hemp extracts is large and growing larger. However, safety concerns have been raised by medical and regulatory agencies. Post marketing surveillance of full spectrum hemp extract (FSHE) products manufactured and distributed by CV Sciences (CVSI) and traded under the brand PlusCBD™ was conducted over a 2-year period (2018-2019). The safety of these products was assessed by analyzing adverse events reports.
From a total of approximately five million product units sold during the 2-year period, 1,429 (0.03%) adverse events (AE) were reported in 1,151 unique customers. Of those, only two were classified as serious AEs. For orally ingested products, the most common types of AEs reported were gastrointestinal (e.g. abdominal discomfort), while for topically applied products, the most reports mentioned dermatological symptoms (e.g. rashes). There has been no evidence of liver toxicity associated with CVSI products.
Based on this longitudinal dataset, the products manufactured using CVSI’s proprietary processes are safe and well tolerated at the recommended doses.”
https://pubmed.ncbi.nlm.nih.gov/32449632/
https://www.tandfonline.com/doi/abs/10.1080/19390211.2020.1767255?journalCode=ijds20

 “Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain.
“Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain. “Cannabidiol (CBD) and cannabigerol (CBG) are Cannabis sativa terpenophenols.
“Cannabidiol (CBD) and cannabigerol (CBG) are Cannabis sativa terpenophenols.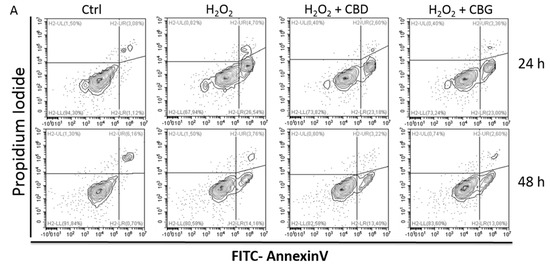
 “In this study cannabidiol (CBD) was administered orally to determine its effects and mechanisms in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS). We hypothesized that 75 mg/kg of oral CBD given for 5 days after initiation of disease would reduce EAE severity through suppression of either the early peripheral immune or late neuroimmune response.
“In this study cannabidiol (CBD) was administered orally to determine its effects and mechanisms in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS). We hypothesized that 75 mg/kg of oral CBD given for 5 days after initiation of disease would reduce EAE severity through suppression of either the early peripheral immune or late neuroimmune response.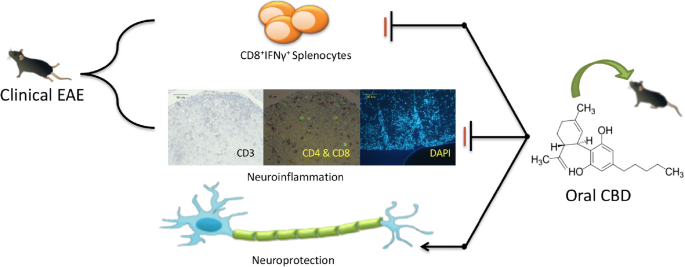
 “Cannabidiol (CBD), the major non-psychoactive constituent of Cannabis sativa L., has gained traction as a potential treatment for intractable chronic pain in many conditions. Clinical evidence suggests that CBD provides therapeutic benefit in certain forms of epilepsy and imparts analgesia in certain conditions, and improves quality of life.
“Cannabidiol (CBD), the major non-psychoactive constituent of Cannabis sativa L., has gained traction as a potential treatment for intractable chronic pain in many conditions. Clinical evidence suggests that CBD provides therapeutic benefit in certain forms of epilepsy and imparts analgesia in certain conditions, and improves quality of life.
 “Medical cannabis (MC) is becoming more and more popular among patients with chronic pain syndromes.
“Medical cannabis (MC) is becoming more and more popular among patients with chronic pain syndromes. “Historical relevance: Cannabis sativa L. (C. sativa) is a plant whose use as a therapeutic agent shares its origins with the first Far East’s human societies. Cannabis has been used not only for recreational purposes, but as a food to obtain textile fibers, to produce hemp paper, to treat many physical and mental disorders.
“Historical relevance: Cannabis sativa L. (C. sativa) is a plant whose use as a therapeutic agent shares its origins with the first Far East’s human societies. Cannabis has been used not only for recreational purposes, but as a food to obtain textile fibers, to produce hemp paper, to treat many physical and mental disorders. “The cannabis plant has been widely researched for many therapeutic indications and found to be effective in many chronic conditions such as epilepsy, neuropathic or chronic pain and more. However, biased opinion against compounds of the plant, regulatory as well as compounding challenges have led to very few approved medicinal products. Those formulations which are approved are dosed several times a day, creating an unmet need for controlled release (CR) formulations of
“The cannabis plant has been widely researched for many therapeutic indications and found to be effective in many chronic conditions such as epilepsy, neuropathic or chronic pain and more. However, biased opinion against compounds of the plant, regulatory as well as compounding challenges have led to very few approved medicinal products. Those formulations which are approved are dosed several times a day, creating an unmet need for controlled release (CR) formulations of  “Two patient case reports are presented describing the use of cannabidiol (CBD) for the symptomatic relief of a lumbar compression fracture and in the mitigation of thoracic discomfort and dysesthesia secondary to a surgically resected meningioma.
“Two patient case reports are presented describing the use of cannabidiol (CBD) for the symptomatic relief of a lumbar compression fracture and in the mitigation of thoracic discomfort and dysesthesia secondary to a surgically resected meningioma.