 “A significant number of epilepsy patients are refractory to conventional antiepileptic drugs. These patients experience considerable neurocognitive impairments that impact their quality of life and ability to function independently. This need for alternative treatment has generated increased interest in cannabis use as a therapeutic option in these patients.
“A significant number of epilepsy patients are refractory to conventional antiepileptic drugs. These patients experience considerable neurocognitive impairments that impact their quality of life and ability to function independently. This need for alternative treatment has generated increased interest in cannabis use as a therapeutic option in these patients.
This review seeks to analyze data presented on the pharmacology, safety, and efficacy of cannabis use in patients with drug-resistant epilepsy (DRE) and to propose any future recommendations regarding its use.
The two foremost phytocannabinoids of cannabis showing anticonvulsant properties are tetrahydrocannabinol (THC) and cannabidiol (CBD).
Due to the psychoactive properties of THC, most studies focused on CBD use in these patients. The use of CBD as an adjunct resulted in decreased seizure frequency, and secondary benefits observed included improvement in mood, alertness and sleep. Adverse events (AEs) reported were drowsiness, diarrhea, increased transaminases and worsening of seizures.
It can safely be concluded that there is a significant benefit in DRE patients using CBD as adjunctive therapy. However, further controlled and adequately powered studies are needed to assess the pharmacokinetics and impact of the long-term use of cannabis.”
https://pubmed.ncbi.nlm.nih.gov/32832296/
“The anticonvulsant properties of cannabis have been reported for several years; however, its use as adjunctive therapy in DRE has increased in recent years. Cannabis mediates the ECS, which affects neuronal excitability. This makes it a superior choice for the adjunctive treatment of DRE patients.”

 “In this study, we investigated the effects of exposition to IC50 dose for 24 h of a new synthetic cannabinoid (CB83) and of phytocannabinoids Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on HT-29 colorectal carcinoma cells. Cell viability and proliferative activity evaluated using the MTT, lactate dehydrogenase (LDH), and CyQUANT assays showed that cell viability was significantly affected when CB83, THC, and CBD were administered to cells.
“In this study, we investigated the effects of exposition to IC50 dose for 24 h of a new synthetic cannabinoid (CB83) and of phytocannabinoids Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on HT-29 colorectal carcinoma cells. Cell viability and proliferative activity evaluated using the MTT, lactate dehydrogenase (LDH), and CyQUANT assays showed that cell viability was significantly affected when CB83, THC, and CBD were administered to cells.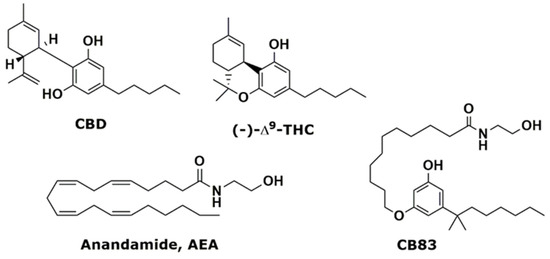
 “Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.
“Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.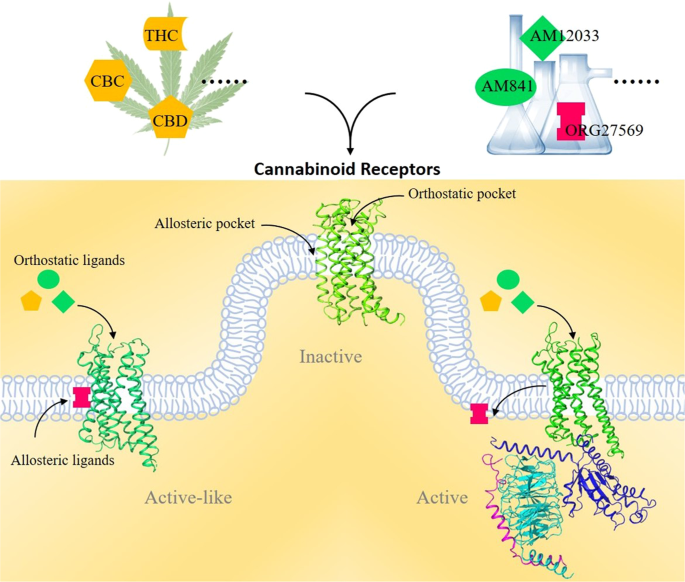
 “In the last few years research into Cannabis and its constituent phytocannabinoids has burgeoned, particularly in the potential application of novel cannabis phytochemicals for the treatment of diverse illnesses related to neurodegeneration and dementia, including Alzheimer’s (AD), Parkinson’s (PD) and Huntington’s disease (HD). To date, these neurological diseases have mostly relied on symptomatological management. However, with an aging population globally, the search for more efficient and disease-modifying treatments that could delay or mitigate disease progression is imperative. In this context, this review aims to present a state of art in the research with cannabinoids and novel cannabinoid-based drug candidates that have been emerged as novel promising alternatives for drug development and innovation in the therapeutics of a number of diseases, especially those related to CNS-disturbance and impairment.”
“In the last few years research into Cannabis and its constituent phytocannabinoids has burgeoned, particularly in the potential application of novel cannabis phytochemicals for the treatment of diverse illnesses related to neurodegeneration and dementia, including Alzheimer’s (AD), Parkinson’s (PD) and Huntington’s disease (HD). To date, these neurological diseases have mostly relied on symptomatological management. However, with an aging population globally, the search for more efficient and disease-modifying treatments that could delay or mitigate disease progression is imperative. In this context, this review aims to present a state of art in the research with cannabinoids and novel cannabinoid-based drug candidates that have been emerged as novel promising alternatives for drug development and innovation in the therapeutics of a number of diseases, especially those related to CNS-disturbance and impairment.” “In recent years, the role of the endocannabinoid system (ECS) in various cardiovascular conditions has been a subject of great interest. The ECS is composed of cannabinoid receptors, their endogenous ligands, also known as endocannabinoids, and enzymes responsible for the synthesis and degradation of endocannabinoids.
“In recent years, the role of the endocannabinoid system (ECS) in various cardiovascular conditions has been a subject of great interest. The ECS is composed of cannabinoid receptors, their endogenous ligands, also known as endocannabinoids, and enzymes responsible for the synthesis and degradation of endocannabinoids. “Thirty years ago, the discovery of a cannabinoid (CB) receptor that interacts with the psychoactive compound in Cannabis led to the identification of anandamide, an endogenous receptor ligand or endocannabinoid. Research on endocannabinoids has since exploded, and additional receptors along with their lipid mediators and signaling pathways continue to be revealed. Specifically, in humans, the release of endocannabinoids from membrane lipids occurs on demand and the signaling process is rapidly attenuated by the breakdown of the ligand suggesting a tight regulation of the endocannabinoid system (ECS). Additionally, the varying distribution of CB receptors between the central nervous system and other tissues allows for the ECS to participate in a wide range of cognitive and physiological processes. Select plant-derived ‘phyto’cannabinoids such as Δ-9-tetrahydrocannabinol (Δ9-THC) bind to the CB receptors and trigger the ECS, and in the case of Δ9-THC, while it has therapeutic value, can also produce detrimental effects. Current research is aimed at the identification of additional phytocannabinoids with minimal psychotropic effects with potential for therapeutic development. Although decades of research on the ECS and its components have expanded our understanding of the mechanisms and implications of endocannabinoid signaling in mammals, it continues to evolve. Here, we provide a brief overview of the ECS and its overlap with other related lipid-mediated signaling pathways.”
“Thirty years ago, the discovery of a cannabinoid (CB) receptor that interacts with the psychoactive compound in Cannabis led to the identification of anandamide, an endogenous receptor ligand or endocannabinoid. Research on endocannabinoids has since exploded, and additional receptors along with their lipid mediators and signaling pathways continue to be revealed. Specifically, in humans, the release of endocannabinoids from membrane lipids occurs on demand and the signaling process is rapidly attenuated by the breakdown of the ligand suggesting a tight regulation of the endocannabinoid system (ECS). Additionally, the varying distribution of CB receptors between the central nervous system and other tissues allows for the ECS to participate in a wide range of cognitive and physiological processes. Select plant-derived ‘phyto’cannabinoids such as Δ-9-tetrahydrocannabinol (Δ9-THC) bind to the CB receptors and trigger the ECS, and in the case of Δ9-THC, while it has therapeutic value, can also produce detrimental effects. Current research is aimed at the identification of additional phytocannabinoids with minimal psychotropic effects with potential for therapeutic development. Although decades of research on the ECS and its components have expanded our understanding of the mechanisms and implications of endocannabinoid signaling in mammals, it continues to evolve. Here, we provide a brief overview of the ECS and its overlap with other related lipid-mediated signaling pathways.”
 “HIV/SIV-associated oral mucosal disease/dysfunction (HAOMD) (gingivitis/periodontitis/salivary adenitis) represents a major comorbidity affecting HIV patients on anti-retroviral therapy.
“HIV/SIV-associated oral mucosal disease/dysfunction (HAOMD) (gingivitis/periodontitis/salivary adenitis) represents a major comorbidity affecting HIV patients on anti-retroviral therapy. “Phytocannabinoids are bioactive natural products found in some flowering
“Phytocannabinoids are bioactive natural products found in some flowering  “Like most modern molecular biology and natural product chemistry, understanding cannabinoid pharmacology centers around molecular interactions, in this case, between the cannabinoids and their putative targets, the G-protein coupled receptors (GPCRs) cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2). Understanding the complex structure and interplay between the partners in this molecular dance is required to understand the mechanism of action of synthetic, endogenous, and phytochemical cannabinoids. This review, with 91 references, surveys our understanding of the structural biology of the cannabinoids and their target receptors including both a critical comparison of the extant crystal structures and the computationally derived homology models, as well as an in-depth discussion about the binding modes of the major cannabinoids. The aim is to assist in situating structural biochemists, synthetic chemists, and molecular biologists who are new to the field of cannabis research.”
“Like most modern molecular biology and natural product chemistry, understanding cannabinoid pharmacology centers around molecular interactions, in this case, between the cannabinoids and their putative targets, the G-protein coupled receptors (GPCRs) cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2). Understanding the complex structure and interplay between the partners in this molecular dance is required to understand the mechanism of action of synthetic, endogenous, and phytochemical cannabinoids. This review, with 91 references, surveys our understanding of the structural biology of the cannabinoids and their target receptors including both a critical comparison of the extant crystal structures and the computationally derived homology models, as well as an in-depth discussion about the binding modes of the major cannabinoids. The aim is to assist in situating structural biochemists, synthetic chemists, and molecular biologists who are new to the field of cannabis research.” “Embase and Pubmed were systematically searched for articles addressing the neuroprotective properties of phytocannabinoids, aside from cannabidiol and Δ9 -tetrahydrocannabinol, including Δ9 -tetrahydrocannabinolic acid (Δ9 -THCA), Δ9 -tetrahydrocannabivarin (Δ9 -THCV), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabichromene (CBC), cannabichromenic acid (CBCA), cannabichromevarin (CBCV), cannabigerol (CBG), cannabigerolic acid (CBGA), cannabigerivarin (CBGV), cannabigerovarinic acid (CBGVA), cannabichromevarinic acid (CBCVA) cannabidivarinic acid (CBDVA) and cannabinol (CBN).
“Embase and Pubmed were systematically searched for articles addressing the neuroprotective properties of phytocannabinoids, aside from cannabidiol and Δ9 -tetrahydrocannabinol, including Δ9 -tetrahydrocannabinolic acid (Δ9 -THCA), Δ9 -tetrahydrocannabivarin (Δ9 -THCV), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabichromene (CBC), cannabichromenic acid (CBCA), cannabichromevarin (CBCV), cannabigerol (CBG), cannabigerolic acid (CBGA), cannabigerivarin (CBGV), cannabigerovarinic acid (CBGVA), cannabichromevarinic acid (CBCVA) cannabidivarinic acid (CBDVA) and cannabinol (CBN).