“The aberrant accumulation of disease-specific protein aggregates accompanying cognitive decline is a pathological hallmark of age-associated neurological disorders, also termed as proteinopathies, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis and multiple sclerosis.
“The aberrant accumulation of disease-specific protein aggregates accompanying cognitive decline is a pathological hallmark of age-associated neurological disorders, also termed as proteinopathies, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis and multiple sclerosis.
Along with oxidative stress and neuroinflammation, disruption in protein homeostasis (proteostasis), a network that constitutes protein surveillance system, plays a pivotal role in the pathobiology of these dementia disorders.
Cannabidiol, a non-psychotropic phytocannabinoid of Cannabis sativa, is known for its pleiotropic neuropharmacological effects on the central nervous system, including the ability to abate oxidative stress, neuroinflammation, and protein misfolding. Over the past years, compelling evidence has documented disease-modifying role of cannabidiol in various preclinical and clinical models of neurological disorders, suggesting the potential therapeutic implications of cannabidiol in these disorders.
Because of its putative role in the proteostasis network in particular, cannabidiol could be a potent modulator for reversing not only age-associated neurodegeneration but also other protein misfolding disorders. However, the current understanding is insufficient to underpin this proposition. In this review, we discuss the potentiality of cannabidiol as a pharmacological modulator of the proteostasis network, highlighting its neuroprotective and aggregates clearing roles in the neurodegenerative disorders.
We anticipate that the current effort will advance our knowledge on the implication of CBD in proteostasis network, opening up a new therapeutic window for ageing proteinopathies.”
https://pubmed.ncbi.nlm.nih.gov/33181336/
“Cannabidiol reduces oxidative stress and neuroinflammation of brain.”
https://www.sciencedirect.com/science/article/pii/S1568163720303445?via%3Dihub

 “Cannabidiol (CBD) is a non-psychoactive phytocannabinoid known for its beneficial effects including antioxidant and anti-inflammatory properties. Moreover, CBD is a compound with antidepressant, anxiolytic, anticonvulsant and antipsychotic effects. Thanks to all these properties, the interest of the scientific community for it has grown.
“Cannabidiol (CBD) is a non-psychoactive phytocannabinoid known for its beneficial effects including antioxidant and anti-inflammatory properties. Moreover, CBD is a compound with antidepressant, anxiolytic, anticonvulsant and antipsychotic effects. Thanks to all these properties, the interest of the scientific community for it has grown. “Cannabinoids have been found to be effective in controlling seizures and the highly purified form of cannabinoid derived for Cannabis sativa . Cannabidiol (CBD) is now approved for Lennox-Gastaut syndrome (LGS) and Dravet syndrome. CBD was used in a 9-year-old boy with LGS (unknown etiology) with very good results. The electroencephalography (EEG) response was very dramatic with near normalization of EEG background and complete control of seizures. The effect of CBD on EEG with such an improvement has not been described previously. Also, this adds to evidence that early intervention in LGS with CBD might be more helpful and improve outcomes.”
“Cannabinoids have been found to be effective in controlling seizures and the highly purified form of cannabinoid derived for Cannabis sativa . Cannabidiol (CBD) is now approved for Lennox-Gastaut syndrome (LGS) and Dravet syndrome. CBD was used in a 9-year-old boy with LGS (unknown etiology) with very good results. The electroencephalography (EEG) response was very dramatic with near normalization of EEG background and complete control of seizures. The effect of CBD on EEG with such an improvement has not been described previously. Also, this adds to evidence that early intervention in LGS with CBD might be more helpful and improve outcomes.” “Cocaine addiction is a global health problem with no approved pharmacotherapies.
“Cocaine addiction is a global health problem with no approved pharmacotherapies. “Multiple therapeutic properties have been attributed to Cannabis sativa. However, further research is required to unveil the medicinal potential of Cannabis and the relationship between biological activity and chemical profile.
“Multiple therapeutic properties have been attributed to Cannabis sativa. However, further research is required to unveil the medicinal potential of Cannabis and the relationship between biological activity and chemical profile. “Objective: To determine the benefit of a tetrahydrocannabinol (THC)-rich cannabis oil on symptoms and quality of life of fibromyalgia patients.
“Objective: To determine the benefit of a tetrahydrocannabinol (THC)-rich cannabis oil on symptoms and quality of life of fibromyalgia patients.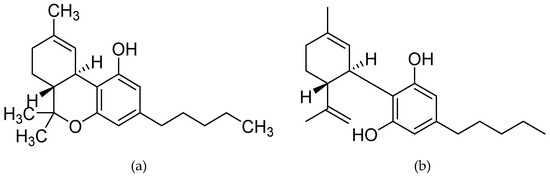
 “These data support the notion that CBD and CBDV act as functional partial agonists on dopamine D2-like receptors in vivo.
“These data support the notion that CBD and CBDV act as functional partial agonists on dopamine D2-like receptors in vivo. “In this proof-of-concept study, the antioxidant activity of phytocannabinoids, namely cannabidiol (CBD) and Δ9- tetrahydrocannabinol (THC), were investigated using an in vitro system of differentiated human neuronal SY-SH5Y cells.
“In this proof-of-concept study, the antioxidant activity of phytocannabinoids, namely cannabidiol (CBD) and Δ9- tetrahydrocannabinol (THC), were investigated using an in vitro system of differentiated human neuronal SY-SH5Y cells.