 “Beta-caryophyllene (BCP) is a flavoring agent, whereas l-arginine (LA) is used as a food supplement.
“Beta-caryophyllene (BCP) is a flavoring agent, whereas l-arginine (LA) is used as a food supplement.
They possess insulinotropic and β cell regeneration activities, respectively.
We assessed the antidiabetic potential of BCP, LA, and its combination in RIN-5F cell lines and diabetic rats.
The results indicated that the combination of BCP with LA showed a significant decrease in glucose absorption and an increase in its uptake in tissues and also an increase in insulin secretion in RIN-5F cells. The combination treatment of BCP with LA showed a significant reduction in glucose, lipid levels, and oxidative stress in pancreatic tissue when compared with the diabetic group. Furthermore, the combination of BCP with LA normalized glucose tolerance and pancreatic cell damage in diabetic rats.
In conclusion, the combinational treatment showed significant potentials in the treatment of type 2 diabetes mellitus.
PRACTICAL APPLICATIONS:
Type 2 diabetes mellitus is the most prevalent chronic metabolic disorder affecting a large population.
Beta-caryophyllene is a CB2 receptor agonist shown to have insulinotropic activity.
l-Arginine is a food supplement that possesses beta-cell regeneration property.
The combination of BCP with LA could work as a potential therapeutic intervention, considering the individual pharmacological activities of each.
We evaluated the antidiabetic activity of the combination of BCP with LA in diabetic rats using ex vivo and in vitro experimentations.
Results from the study revealed that the combination of BCP with LA showed a significant (p < .001) reduction in glucose and lipid levels as compared to individual treatment. In vitro study also supports the diabetic potential of the combination of BCP with LA in the glucose-induced insulin secretion in RIN-5F cell lines.
The study indicates a therapeutic approach to treat T2DM by BCP and LA combination as food and dietary supplement.”
https://www.ncbi.nlm.nih.gov/pubmed/31997410
https://onlinelibrary.wiley.com/doi/abs/10.1111/jfbc.13156
 “The rationale of this study was to assess occurrence of withdrawal symptoms induced by abrupt cessation of cannabidiol (CBD) after prolonged administration in healthy volunteers.
“The rationale of this study was to assess occurrence of withdrawal symptoms induced by abrupt cessation of cannabidiol (CBD) after prolonged administration in healthy volunteers.
 “The use of medical cannabis in children is rapidly growing.
“The use of medical cannabis in children is rapidly growing. “Beta-caryophyllene (BCP) is a flavoring agent, whereas l-arginine (LA) is used as a food supplement.
“Beta-caryophyllene (BCP) is a flavoring agent, whereas l-arginine (LA) is used as a food supplement. “Endo-, phyto- and synthetic cannabinoids have been proposed as promising anti-cancer agents able to impair cancer cells’ behavior without affecting their non-transformed counterparts.
“Endo-, phyto- and synthetic cannabinoids have been proposed as promising anti-cancer agents able to impair cancer cells’ behavior without affecting their non-transformed counterparts.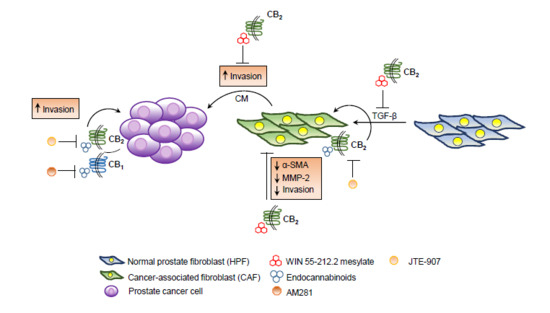
 “Given the growing challenges in chronic pain management coupled with the ongoing consequences of the opioid epidemic, pain management practitioners are looking into more effective, innovative, and safer alternatives to treat pain.
“Given the growing challenges in chronic pain management coupled with the ongoing consequences of the opioid epidemic, pain management practitioners are looking into more effective, innovative, and safer alternatives to treat pain. “Osteoarticular equine disease is a common cause of malady; in general, its therapy is supported on steroids and nonsteroidal anti-inflammatories. Nevertheless, many side effects may develop when these drugs are administered. Nowadays, the use of new alternatives for this pathology attention is demanded; in that sense,
“Osteoarticular equine disease is a common cause of malady; in general, its therapy is supported on steroids and nonsteroidal anti-inflammatories. Nevertheless, many side effects may develop when these drugs are administered. Nowadays, the use of new alternatives for this pathology attention is demanded; in that sense,  “Cannabis sativa L. is an ancient medicinal plant wherefrom over 120 cannabinoids are extracted. In the past two decades, there has been increasing interest in the therapeutic potential of cannabis-based treatments for neurological disorders such as epilepsy, and there is now evidence for the medical use of cannabis and its effectiveness for a wide range of diseases.
“Cannabis sativa L. is an ancient medicinal plant wherefrom over 120 cannabinoids are extracted. In the past two decades, there has been increasing interest in the therapeutic potential of cannabis-based treatments for neurological disorders such as epilepsy, and there is now evidence for the medical use of cannabis and its effectiveness for a wide range of diseases. “Controlled and open label trials have demonstrated efficacy of
“Controlled and open label trials have demonstrated efficacy of