“Coronavirus (SARS-CoV-2), the causative agent of the Covid-19 pandemic has proved itself as the deadliest pathogen. A major portion of the population has become susceptible to this strain. Scientists are pushing their limits to formulate a vaccine against Covid-19 with the least side effects.
Although the recent discoveries of vaccines have shown some relief from the covid infection rate, however, physical fatigue, mental abnormalities, inflammation and other multiple organ damages are arising as post-Covid symptoms. The long-term effects of these symptoms are massive. Patients with such symptoms are known as long-haulers and treatment strategy against this condition is still unknown.
In this study, we tried to explore a strategy to deal with the post-Covid symptoms. We targeted three human proteins namely ACE2, Interleukin-6, Transmembrane serine protease and NRP1 which are already reported to be damaged via Covid-19 proteins and upregulated in the post-Covid stage. Our target plant in this study is Cannabis (popularly known as ‘Ganja’ in India).
The molecular docking and simulation studies revealed that Cannabidiol (CBD) and Cannabivarin (CVN) obtained from Cannabis can bind to post-Covid symptoms related central nervous system (CNS) proteins and downregulate them which can be beneficial in post-covid symptoms treatment strategy. Thus we propose Cannabis as an important therapeutic plant against post-Covid symptoms.”
https://pubmed.ncbi.nlm.nih.gov/33810774/
https://www.tandfonline.com/doi/abs/10.1080/07391102.2021.1905556?journalCode=tbsd20

 “Cannabis sativa
“Cannabis sativa “Autophagy is a “self-degradation” process whereby malfunctioned cytoplasmic constituents and protein aggregates are engulfed by a vesicle called the autophagosome, and subsequently degraded by the lysosome. Autophagy plays a crucial role in sustaining protein homeostasis and can be an alternative source of energy under detrimental circumstances. Studies have demonstrated a paradoxical function for autophagy in cancer, displaying both tumour suppressive and tumour promotive roles. In early phases of tumour development autophagy promotes cancer cell death. In later phases, autophagy enables cancer cells to survive and withstand therapy.
“Autophagy is a “self-degradation” process whereby malfunctioned cytoplasmic constituents and protein aggregates are engulfed by a vesicle called the autophagosome, and subsequently degraded by the lysosome. Autophagy plays a crucial role in sustaining protein homeostasis and can be an alternative source of energy under detrimental circumstances. Studies have demonstrated a paradoxical function for autophagy in cancer, displaying both tumour suppressive and tumour promotive roles. In early phases of tumour development autophagy promotes cancer cell death. In later phases, autophagy enables cancer cells to survive and withstand therapy. 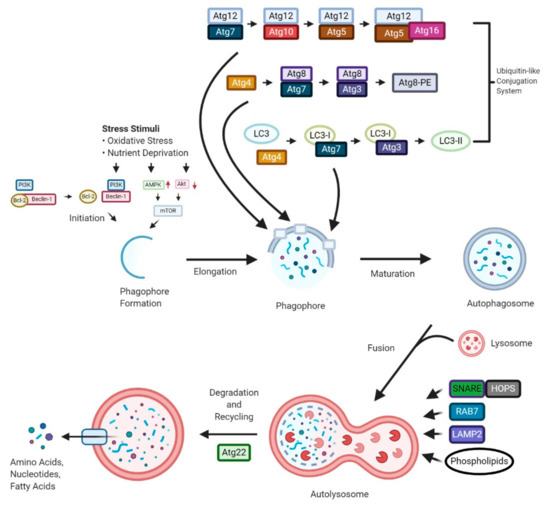
 “The rapid spread of COVID-19 underscores the need for new treatments.
“The rapid spread of COVID-19 underscores the need for new treatments. “Inflammasomes are cytoplasmic inflammatory signaling protein complexes that detect microbial materials, sterile inflammatory insults, and certain host-derived elements. Inflammasomes, once activated, promote caspase-1-mediated maturation and secretion of pro-inflammatory cytokines, interleukin (IL)-1β and IL-18, leading to pyroptosis. Current advances in inflammasome research support their involvement in the development of chronic inflammatory disorders in contrast to their role in regulating innate immunity.
“Inflammasomes are cytoplasmic inflammatory signaling protein complexes that detect microbial materials, sterile inflammatory insults, and certain host-derived elements. Inflammasomes, once activated, promote caspase-1-mediated maturation and secretion of pro-inflammatory cytokines, interleukin (IL)-1β and IL-18, leading to pyroptosis. Current advances in inflammasome research support their involvement in the development of chronic inflammatory disorders in contrast to their role in regulating innate immunity. “Background: Breast Cancer (BC), a common death-causing disease and the deadliest cancer next to lung cancer, is characterized by an abnormal growth of cells in the tissues of the breast. BC chemotherapy is marked by targeting the activities of some receptors such as Estrogen Receptor alpha (ER-α). At present, one of the most commonly used and approved marketed therapeutic drug for BC is tamoxifen. Despite the short term success of tamoxifen usage, its long time treatment has been associated with significant side effects. Therefore, there is a pressing need for the development of novel anti-estrogens for the prevention and treatment of BC.
“Background: Breast Cancer (BC), a common death-causing disease and the deadliest cancer next to lung cancer, is characterized by an abnormal growth of cells in the tissues of the breast. BC chemotherapy is marked by targeting the activities of some receptors such as Estrogen Receptor alpha (ER-α). At present, one of the most commonly used and approved marketed therapeutic drug for BC is tamoxifen. Despite the short term success of tamoxifen usage, its long time treatment has been associated with significant side effects. Therefore, there is a pressing need for the development of novel anti-estrogens for the prevention and treatment of BC.