“Cannabinoid-based medications possess unique multimodal analgesic mechanisms of action, modulating diverse pain targets.
Cannabinoids are classified based on their origin into three categories: endocannabinoids (present endogenously in human tissues), phytocannabinoids (plant derived) and synthetic cannabinoids (pharmaceutical). Cannabinoids exert an analgesic effect, peculiarly in hyperalgesia, neuropathic pain and inflammatory states.
Endocannabinoids are released on demand from postsynaptic terminals and travels retrograde to stimulate cannabinoids receptors on presynaptic terminals, inhibiting the release of excitatory neurotransmitters. Cannabinoids (endogenous and phytocannabinoids) produce analgesia by interacting with cannabinoids receptors type 1 and 2 (CB1 and CB2), as well as putative non-CB1/CB2 receptors; G protein-coupled receptor 55, and transient receptor potential vanilloid type-1. Moreover, they modulate multiple peripheral, spinal and supraspinal nociception pathways.
Cannabinoids-opioids cross-modulation and synergy contribute significantly to tolerance and antinociceptive effects of cannabinoids. This narrative review evaluates cannabinoids’ diverse mechanisms of action as it pertains to nociception modulation relevant to the practice of anesthesiologists and pain medicine physicians.”
https://pubmed.ncbi.nlm.nih.gov/33239391/
https://rapm.bmj.com/content/early/2020/11/24/rapm-2020-102114

 “The potential therapeutic use of some Cannabis sativa plant compounds has been attracting great interest, especially for managing neuropsychiatric disorders due to the relative lack of efficacy of the current treatments.
“The potential therapeutic use of some Cannabis sativa plant compounds has been attracting great interest, especially for managing neuropsychiatric disorders due to the relative lack of efficacy of the current treatments.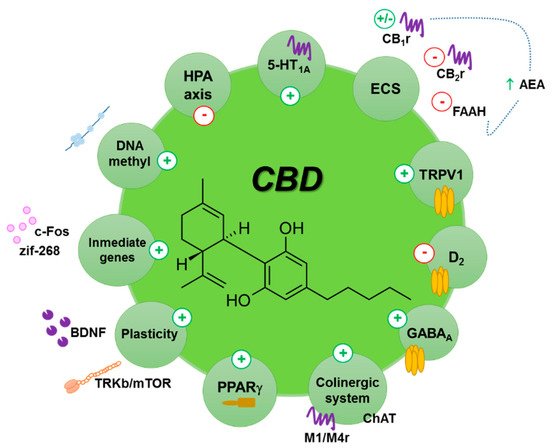
 “The COVID-19 pandemic caused by SARS-CoV-2 is a deadly disease afflicting millions. The pandemic continues affecting population due to nonavailability of drugs and vaccines. The pathogenesis and complications of infection mainly involve hyperimmune-inflammatory responses. Thus, therapeutic strategies rely on repurposing of drugs aimed at reducing infectivity and inflammation and modulate immunity favourably.
“The COVID-19 pandemic caused by SARS-CoV-2 is a deadly disease afflicting millions. The pandemic continues affecting population due to nonavailability of drugs and vaccines. The pathogenesis and complications of infection mainly involve hyperimmune-inflammatory responses. Thus, therapeutic strategies rely on repurposing of drugs aimed at reducing infectivity and inflammation and modulate immunity favourably.
 “The endocannabinoid (eCB) system encompasses the eCBs anandamide and 2-arachidonoylglycerol, their anabolic/catabolic enzymes, and the cannabinoid CB1 and CB2 receptors. Its expansion to include several eCB-like lipid mediators, their metabolic enzymes, and their molecular targets, forms the endocannabinoidome (eCBome).
“The endocannabinoid (eCB) system encompasses the eCBs anandamide and 2-arachidonoylglycerol, their anabolic/catabolic enzymes, and the cannabinoid CB1 and CB2 receptors. Its expansion to include several eCB-like lipid mediators, their metabolic enzymes, and their molecular targets, forms the endocannabinoidome (eCBome). “Recently, cannabinoids, such as cannabidiol (CBD) and Δ9 -tetrahydrocannabinol (THC), have been the subject of intensive research and heavy scrutiny. Cannabinoids encompass a wide array of organic molecules, including those that are physiologically produced in humans, synthesized in laboratories, and extracted primarily from the Cannabis sativa plant. These organic molecules share similarities in their chemical structures as well as in their protein binding profiles. However, pronounced differences do exist in their mechanisms of action and clinical applications, which will be briefly compared and contrasted in this review. The mechanism of action of CBD and its potential applications in cancer therapy will be the major focus of this review article.”
“Recently, cannabinoids, such as cannabidiol (CBD) and Δ9 -tetrahydrocannabinol (THC), have been the subject of intensive research and heavy scrutiny. Cannabinoids encompass a wide array of organic molecules, including those that are physiologically produced in humans, synthesized in laboratories, and extracted primarily from the Cannabis sativa plant. These organic molecules share similarities in their chemical structures as well as in their protein binding profiles. However, pronounced differences do exist in their mechanisms of action and clinical applications, which will be briefly compared and contrasted in this review. The mechanism of action of CBD and its potential applications in cancer therapy will be the major focus of this review article.”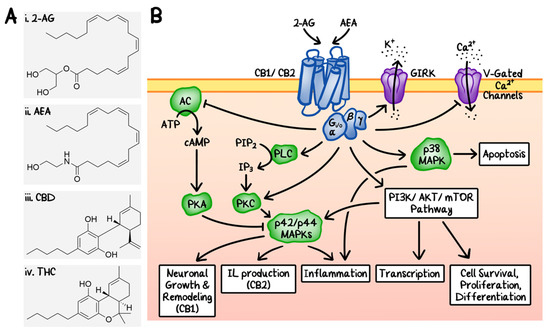
 “Introduction and objectives: Previous studies reveal conflicting data on the effect of cannabis use in patients with cirrhosis. This research evaluates the impact of cannabis on hepatic decompensation, health care utilization, and mortality in patients with cirrhosis.
“Introduction and objectives: Previous studies reveal conflicting data on the effect of cannabis use in patients with cirrhosis. This research evaluates the impact of cannabis on hepatic decompensation, health care utilization, and mortality in patients with cirrhosis. “(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene widely distributed in the plant kingdom, where it contributes a unique aroma to essential oils and has a pivotal role in the survival and evolution of higher plants.
“(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene widely distributed in the plant kingdom, where it contributes a unique aroma to essential oils and has a pivotal role in the survival and evolution of higher plants. “Cocaine addiction is a global health problem with no approved pharmacotherapies.
“Cocaine addiction is a global health problem with no approved pharmacotherapies. “Multiple therapeutic properties have been attributed to Cannabis sativa. However, further research is required to unveil the medicinal potential of Cannabis and the relationship between biological activity and chemical profile.
“Multiple therapeutic properties have been attributed to Cannabis sativa. However, further research is required to unveil the medicinal potential of Cannabis and the relationship between biological activity and chemical profile.