 “This study investigated the effects of adolescent exposure to the main psychoactive component of cannabis, ∆9-tetrahydrocannabinol (THC), in the reinforcing properties of nicotine in adult male mice. Possible alterations in relapse to nicotine-seeking behaviour in adult animals due to THC adolescent exposure were also evaluated.
“This study investigated the effects of adolescent exposure to the main psychoactive component of cannabis, ∆9-tetrahydrocannabinol (THC), in the reinforcing properties of nicotine in adult male mice. Possible alterations in relapse to nicotine-seeking behaviour in adult animals due to THC adolescent exposure were also evaluated.
RESULTS:
Adolescent THC treatment did not modify acquisition and extinction of nicotine self-administration in adulthood. Moreover, THC exposure did not alter relapse to nicotine seeking induced by stress or nicotine-associated cues.
CONCLUSIONS:
These results suggest that a history of exposure to THC during adolescence under these particular conditions does not modify the reinforcing effects and seeking behaviour of nicotine in the adult period.”
https://www.ncbi.nlm.nih.gov/pubmed/31858159
https://link.springer.com/article/10.1007%2Fs00213-019-05416-8

 “The most bioactive ingredient of
“The most bioactive ingredient of  “Pantothenate kinase-associated neurodegeneration is characterized by severe, progressive dystonia. This study aims to describe the reported usage of cannabis products among children with pantothenate kinase-associated neurodegeneration.
“Pantothenate kinase-associated neurodegeneration is characterized by severe, progressive dystonia. This study aims to describe the reported usage of cannabis products among children with pantothenate kinase-associated neurodegeneration. “Chronic pain affects a significant percentage of the United States population, and available pain medications like opioids have drawbacks that make long-term use untenable.
“Chronic pain affects a significant percentage of the United States population, and available pain medications like opioids have drawbacks that make long-term use untenable. “There is considerable interest in the use of cannabinoids for symptom control in palliative care, but there is little high-quality evidence to guide clinical practice.
“There is considerable interest in the use of cannabinoids for symptom control in palliative care, but there is little high-quality evidence to guide clinical practice. “A 64 year old male heating engineer was investigated for a persistent cough and found to have epithelioid mesothelioma with pleural effusion, lung nodules and increased thoracic lymph nodes. He declined standard of care treatment following his own research and he was enrolled in a named patient programme of IMM-101. He was advised to correct his low vitamin D3 level and to start using anti-inflammatories such as aspirin, bromelain and low dose Naltrexone. At review one year later a CT scan showed no change and he continued on the regimen. Four years after the diagnosis a CT scan showed that there was a modest but definite progression of the left malignant pleural thickening, and a new right-sided effusion, enlargement of several intrathoracic nodes which had been noted on the early scans. The chest wall lump eventually broke down and required local radiotherapy. He then developed abdominal pain and found to have peritoneal disease. Last year he obtained the
“A 64 year old male heating engineer was investigated for a persistent cough and found to have epithelioid mesothelioma with pleural effusion, lung nodules and increased thoracic lymph nodes. He declined standard of care treatment following his own research and he was enrolled in a named patient programme of IMM-101. He was advised to correct his low vitamin D3 level and to start using anti-inflammatories such as aspirin, bromelain and low dose Naltrexone. At review one year later a CT scan showed no change and he continued on the regimen. Four years after the diagnosis a CT scan showed that there was a modest but definite progression of the left malignant pleural thickening, and a new right-sided effusion, enlargement of several intrathoracic nodes which had been noted on the early scans. The chest wall lump eventually broke down and required local radiotherapy. He then developed abdominal pain and found to have peritoneal disease. Last year he obtained the  “This observational study examined the acute cognitive effects of cannabis.
“This observational study examined the acute cognitive effects of cannabis. “Cannabis sativa L. is a plant long used for its textile fibers, seed oil, and oleoresin with medicinal and psychoactive properties. It is the main source of phytocannabinoids, with over 100 compounds detected so far. In recent years, a lot of attention has been given to the main phytochemicals present in Cannabis sativa L., namely,
“Cannabis sativa L. is a plant long used for its textile fibers, seed oil, and oleoresin with medicinal and psychoactive properties. It is the main source of phytocannabinoids, with over 100 compounds detected so far. In recent years, a lot of attention has been given to the main phytochemicals present in Cannabis sativa L., namely, 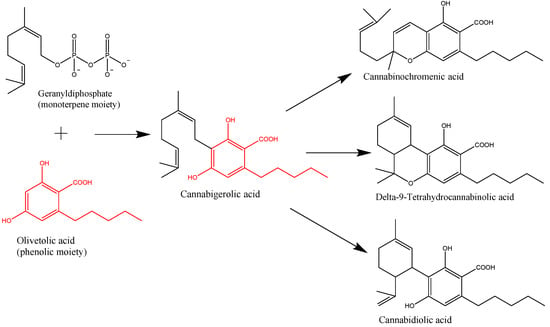

 “Use of
“Use of