 “Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.
“Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.
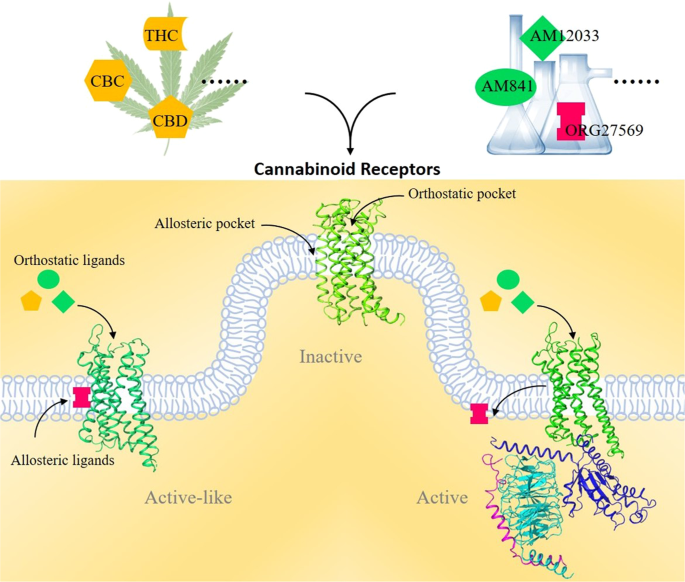
Both exogenous and endogenous CBs carry out a variety of physiological functions by engaging with two CB receptors, the CB1 and CB2 receptors, in the human endocannabinoid system (ECS). Both CB1 and CB2 are G protein-coupled receptors that share a 7-transmembrane (7TM) topology. CB1, known as the central CB receptor, is mainly distributed in the brain, spinal cord, and peripheral nervous system. CB1 activation in the human body typically promotes the release of neurotransmitters, controls pain and memory learning, and regulates metabolism and the cardiovascular system.
Clinically, CB1 is a direct drug target for drug addiction, neurodegenerative diseases, pain, epilepsy, and obesity. Unlike the exclusive expression of CB1 in the nervous system, CB2 is mainly distributed in peripheral immune cells. Selective CB2 agonists would have therapeutic potential in the treatment of inflammation and pain and avoid side effects caused by currently used clinical drugs.
Although significant progress has been made in developing agonists toward CB receptors, efficient clinical drugs targeting CB receptors remain lacking due to their complex signaling mechanisms. The recent structural elucidation of CB receptors has greatly aided our understanding of the activation and signal transduction mechanisms of CB receptors.
Recent structural characterizations of CB receptors will greatly facilitate the design of new ligands to modulate the selective functions of CB receptors. Notably, the CBD was approved by the Food and Drug Administration (FDA) in 2018 to treat epilepsy. We now look forward to more drugs targeting these two CB receptors for clinical usage in the near future.”

 “HIV/SIV-associated oral mucosal disease/dysfunction (HAOMD) (gingivitis/periodontitis/salivary adenitis) represents a major comorbidity affecting HIV patients on anti-retroviral therapy.
“HIV/SIV-associated oral mucosal disease/dysfunction (HAOMD) (gingivitis/periodontitis/salivary adenitis) represents a major comorbidity affecting HIV patients on anti-retroviral therapy. “Deposition of amyloid-beta (Aβ) peptide in the brain is the leading source of the onset and progression of Alzheimer’s disease (AD). Recent studies have suggested that anti-amyloidogenic agents may be a suitable therapeutic strategy for AD.
“Deposition of amyloid-beta (Aβ) peptide in the brain is the leading source of the onset and progression of Alzheimer’s disease (AD). Recent studies have suggested that anti-amyloidogenic agents may be a suitable therapeutic strategy for AD. “Objectives: To investigate the action of cannabinoids on spasticity and pain in secondary progressive multiple sclerosis, by means of neurophysiological indexes.
“Objectives: To investigate the action of cannabinoids on spasticity and pain in secondary progressive multiple sclerosis, by means of neurophysiological indexes. “Hempseeds, the edible fruits of the Cannabis sativa L. plant, were initially considered a by-product of the hemp technical fibre industry. Nowadays, following the restorationing of the cultivation of C. sativa L. plants containing an amount of delta-9-tetrahydrocannabinol (THC) <0.3% or 0.2% (industrial hemp) there is a growing interest for the hempseeds production due to their high nutritional value and functional features.
“Hempseeds, the edible fruits of the Cannabis sativa L. plant, were initially considered a by-product of the hemp technical fibre industry. Nowadays, following the restorationing of the cultivation of C. sativa L. plants containing an amount of delta-9-tetrahydrocannabinol (THC) <0.3% or 0.2% (industrial hemp) there is a growing interest for the hempseeds production due to their high nutritional value and functional features.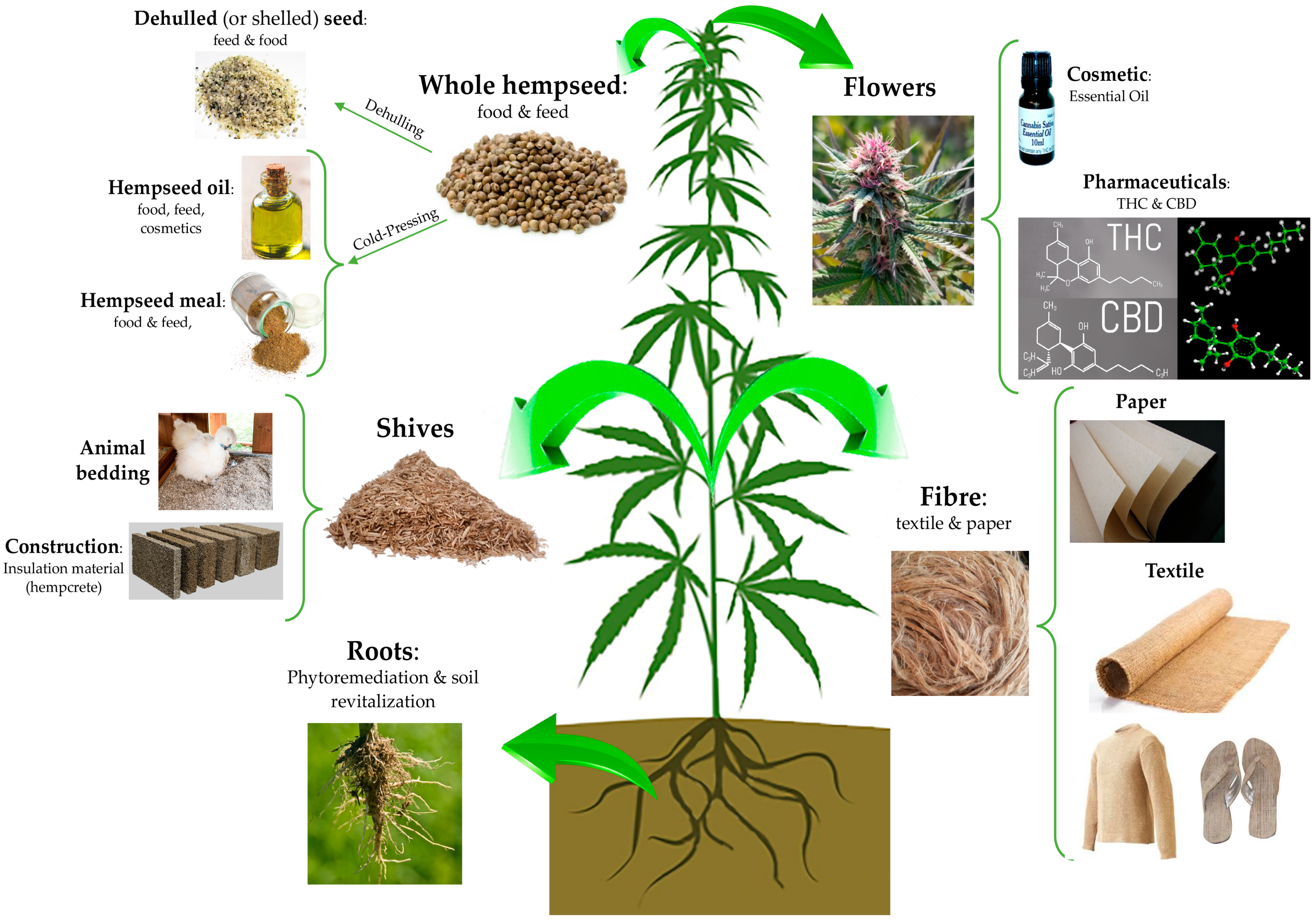
 “Acute Respiratory Distress Syndrome (ARDS) is a life-threatening complication that can ensue following Staphylococcus aureus infection. The enterotoxin produced by these bacteria (SEB) acts as a superantigen thereby activating a large proportion of T cells leading to cytokine storm and severe lung injury.
“Acute Respiratory Distress Syndrome (ARDS) is a life-threatening complication that can ensue following Staphylococcus aureus infection. The enterotoxin produced by these bacteria (SEB) acts as a superantigen thereby activating a large proportion of T cells leading to cytokine storm and severe lung injury. “Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis.
“Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis. “Extracts from the cannabis plant can dramatically improve the health of children suffering from refractory epilepsies such as Dravet syndrome.
“Extracts from the cannabis plant can dramatically improve the health of children suffering from refractory epilepsies such as Dravet syndrome. “Scientific research on how consumption of whole, natural Cannabis flower affects low mood and behavioral motivations more generally is largely nonexistent, and few studies to date have measured how common and commercially available Cannabis flower used in vivo may affect the experience of “depression” in real-time.
“Scientific research on how consumption of whole, natural Cannabis flower affects low mood and behavioral motivations more generally is largely nonexistent, and few studies to date have measured how common and commercially available Cannabis flower used in vivo may affect the experience of “depression” in real-time. “Studies have reported changes in the endocannabinoid system in the brain of patients with Alzheimer’s disease (AD), playing a role in the pathophysiology of AD. Cannabinoids have been shown to have neuroprotective properties, reduce neuroinflammation, and enhance neurogenesis. Evidence suggests that the utilization of marijuana products containing both tetrahydrocannabinol (THC) and cannabidiol (CBD) or CBD alone have been effective and safe for use in older people with agitation associated with dementia.
“Studies have reported changes in the endocannabinoid system in the brain of patients with Alzheimer’s disease (AD), playing a role in the pathophysiology of AD. Cannabinoids have been shown to have neuroprotective properties, reduce neuroinflammation, and enhance neurogenesis. Evidence suggests that the utilization of marijuana products containing both tetrahydrocannabinol (THC) and cannabidiol (CBD) or CBD alone have been effective and safe for use in older people with agitation associated with dementia.