 “Posttraumatic stress disorder (PTSD) may stem from the formation of aberrant and enduring aversive memories. Some PTSD patients have recreationally used Cannabis, probably aiming at relieving their symptomatology.
“Posttraumatic stress disorder (PTSD) may stem from the formation of aberrant and enduring aversive memories. Some PTSD patients have recreationally used Cannabis, probably aiming at relieving their symptomatology.
Here, we seek to review and discuss the effects of THC on aversive memory extinction and anxiety in healthy humans and PTSD patients.
Results: At low doses, THC can enhance the extinction rate and reduce anxiety responses. Both effects involve the activation of cannabinoid type-1 receptors in discrete components of the corticolimbic circuitry, which could couterbalance the low “endocannabinoid tonus” reported in PTSD patients. The advantage of associating CBD with THC to attenuate anxiety while minimizing the potential psychotic or anxiogenic effect produced by high doses of THC has been reported. The effects of THC either alone or combined with CBD on aversive memory reconsolidation, however, are still unknown.
Conclusions: Current evidence from healthy humans and PTSD patients supports the THC value to suppress anxiety and aversive memory expression without producing significant adverse effects if used in low doses or when associated with CBD. Future studies are guaranteed to address open questions related to their dose ratios, administration routes, pharmacokinetic interactions, sex-dependent differences, and prolonged efficacy.”
https://pubmed.ncbi.nlm.nih.gov/32842985/
“Altogether, the findings encourage future controlled studies evaluating the effects of low doses of THC to attenuate aversive/traumatic memory expression in PTSD patients.”
https://bmcpsychiatry.biomedcentral.com/articles/10.1186/s12888-020-02813-8

 “In this study, we investigated the effects of exposition to IC50 dose for 24 h of a new synthetic cannabinoid (CB83) and of phytocannabinoids Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on HT-29 colorectal carcinoma cells. Cell viability and proliferative activity evaluated using the MTT, lactate dehydrogenase (LDH), and CyQUANT assays showed that cell viability was significantly affected when CB83, THC, and CBD were administered to cells.
“In this study, we investigated the effects of exposition to IC50 dose for 24 h of a new synthetic cannabinoid (CB83) and of phytocannabinoids Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on HT-29 colorectal carcinoma cells. Cell viability and proliferative activity evaluated using the MTT, lactate dehydrogenase (LDH), and CyQUANT assays showed that cell viability was significantly affected when CB83, THC, and CBD were administered to cells.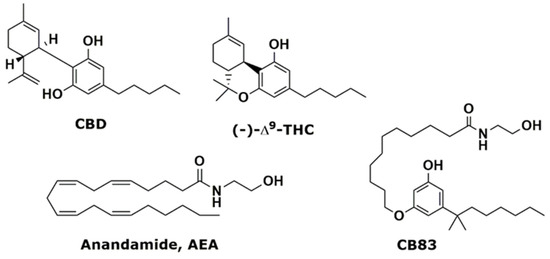
 “Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.
“Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.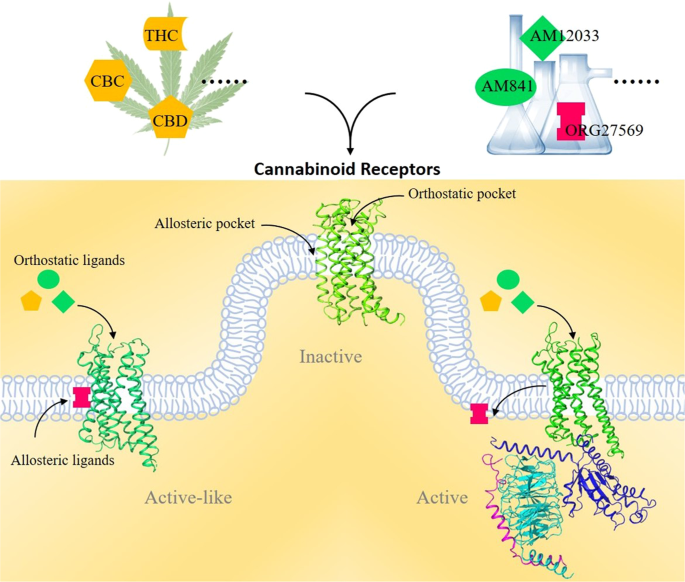
 “HIV/SIV-associated oral mucosal disease/dysfunction (HAOMD) (gingivitis/periodontitis/salivary adenitis) represents a major comorbidity affecting HIV patients on anti-retroviral therapy.
“HIV/SIV-associated oral mucosal disease/dysfunction (HAOMD) (gingivitis/periodontitis/salivary adenitis) represents a major comorbidity affecting HIV patients on anti-retroviral therapy. “Deposition of amyloid-beta (Aβ) peptide in the brain is the leading source of the onset and progression of Alzheimer’s disease (AD). Recent studies have suggested that anti-amyloidogenic agents may be a suitable therapeutic strategy for AD.
“Deposition of amyloid-beta (Aβ) peptide in the brain is the leading source of the onset and progression of Alzheimer’s disease (AD). Recent studies have suggested that anti-amyloidogenic agents may be a suitable therapeutic strategy for AD. “Objectives: To investigate the action of cannabinoids on spasticity and pain in secondary progressive multiple sclerosis, by means of neurophysiological indexes.
“Objectives: To investigate the action of cannabinoids on spasticity and pain in secondary progressive multiple sclerosis, by means of neurophysiological indexes. “Hempseeds, the edible fruits of the Cannabis sativa L. plant, were initially considered a by-product of the hemp technical fibre industry. Nowadays, following the restorationing of the cultivation of C. sativa L. plants containing an amount of delta-9-tetrahydrocannabinol (THC) <0.3% or 0.2% (industrial hemp) there is a growing interest for the hempseeds production due to their high nutritional value and functional features.
“Hempseeds, the edible fruits of the Cannabis sativa L. plant, were initially considered a by-product of the hemp technical fibre industry. Nowadays, following the restorationing of the cultivation of C. sativa L. plants containing an amount of delta-9-tetrahydrocannabinol (THC) <0.3% or 0.2% (industrial hemp) there is a growing interest for the hempseeds production due to their high nutritional value and functional features.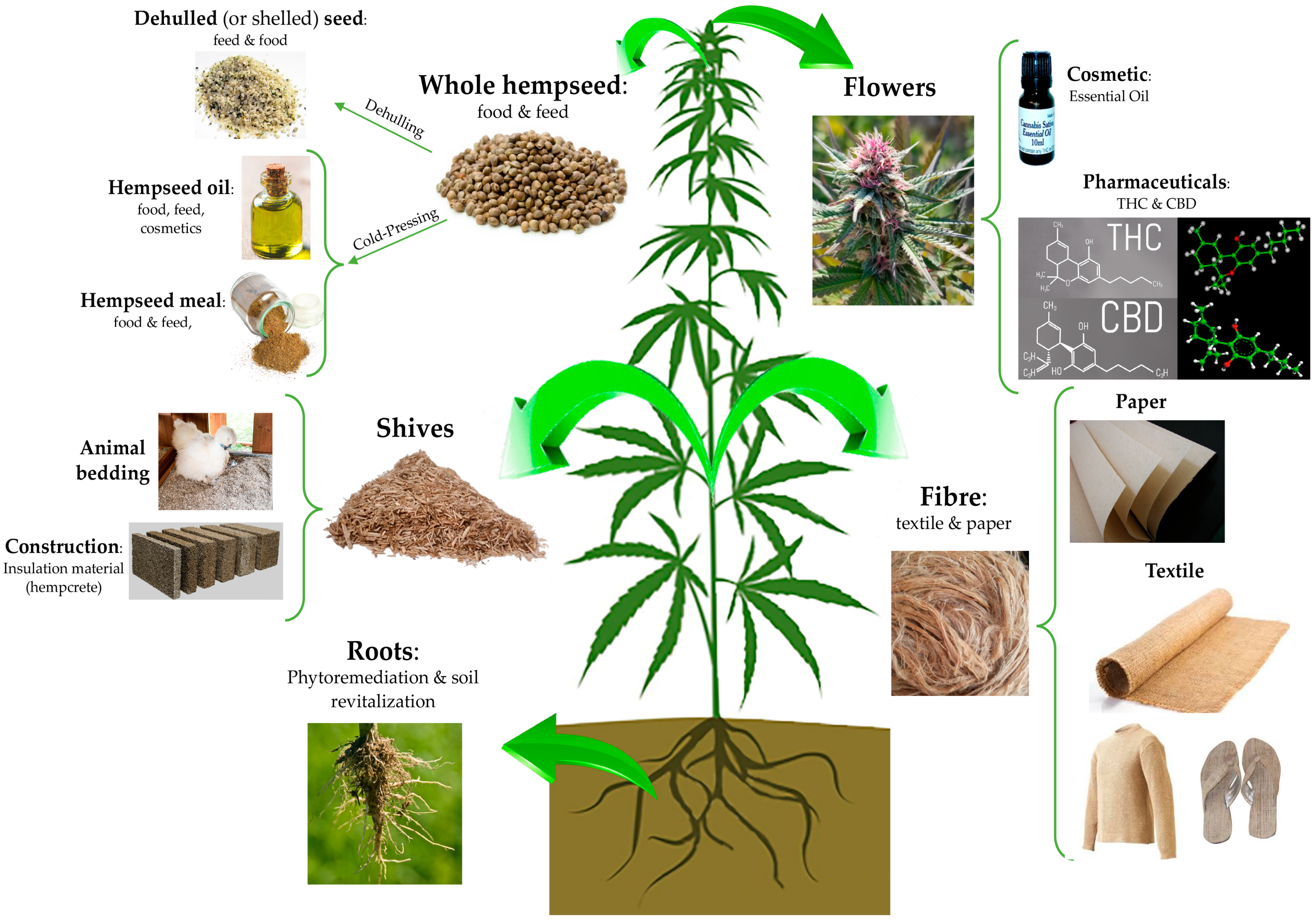
 “Acute Respiratory Distress Syndrome (ARDS) is a life-threatening complication that can ensue following Staphylococcus aureus infection. The enterotoxin produced by these bacteria (SEB) acts as a superantigen thereby activating a large proportion of T cells leading to cytokine storm and severe lung injury.
“Acute Respiratory Distress Syndrome (ARDS) is a life-threatening complication that can ensue following Staphylococcus aureus infection. The enterotoxin produced by these bacteria (SEB) acts as a superantigen thereby activating a large proportion of T cells leading to cytokine storm and severe lung injury. “Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis.
“Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis. “Extracts from the cannabis plant can dramatically improve the health of children suffering from refractory epilepsies such as Dravet syndrome.
“Extracts from the cannabis plant can dramatically improve the health of children suffering from refractory epilepsies such as Dravet syndrome.