
In the current study, we examined the role of the type 2 cannabinoid receptor (CB2R) which is expressed on nearly all immune cells and demonstrated that absence of the CB2R on donor CD4+ or CD8+ T cells, or administration of a selective CB2R pharmacological antagonist, exacerbated acute GVHD lethality. This was accompanied primarily by the expansion of proinflammatory CD8+ T cells indicating that constitutive CB2R expression on T cells preferentially regulated CD8+ T cell alloreactivity. Using a novel CB2R-EGFP reporter mouse, we observed significant loss of CB2R expression on T cells, but not macrophages, during acute GVHD, indicative of differential alterations in receptor expression under inflammatory conditions.
Therapeutic targeting of the CB2R with the agonists, tetrahydrocannabinol (THC) and JWH-133, revealed that only THC mitigated lethal T cell-mediated acute GVHD. Conversely, only JWH-133 was effective in a sclerodermatous chronic GVHD model where macrophages contribute to disease biology. In vitro, both THC and JWH-133 induced arrestin recruitment and ERK phosphorylation via CB2R, but THC had no effect on CB2R-mediated inhibition of adenylyl cyclase.
These studies demonstrate that the CB2R plays a critical role in the regulation of GVHD and suggest that effective therapeutic targeting is dependent upon agonist signaling characteristics and receptor selectivity in conjunction with the composition of pathogenic immune effector cells.”

 “Cannabinoids have long been used for their psychotropic and possible medical properties of symptom relief. In the past few years, a vast literature shows that cannabinoids are neuroprotective under different pathological situations.
“Cannabinoids have long been used for their psychotropic and possible medical properties of symptom relief. In the past few years, a vast literature shows that cannabinoids are neuroprotective under different pathological situations. “Pharmaceutically purified oral cannabidiol (CBD) has been recently approved by the US Food and Drug Administration and European Medicines Agency as treatment of seizures associated with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS), which are severe and difficult-to-treat developmental and epileptic encephalopathies with onset in early childhood.
“Pharmaceutically purified oral cannabidiol (CBD) has been recently approved by the US Food and Drug Administration and European Medicines Agency as treatment of seizures associated with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS), which are severe and difficult-to-treat developmental and epileptic encephalopathies with onset in early childhood. “Rett syndrome (RTT) is a rare neurologic disorder, characterized by severe behavioural and physiological symptoms. RTT is caused by mutations in the MECP2 gene in about 95% of cases and to date no cure is available.
“Rett syndrome (RTT) is a rare neurologic disorder, characterized by severe behavioural and physiological symptoms. RTT is caused by mutations in the MECP2 gene in about 95% of cases and to date no cure is available. “Diabetes mellitus (DM), a metabolic disorder is one of the most prevalent chronic diseases worldwide across developed as well as developing nations. Hyperglycemia is the core feature of the type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), following insulin deficiency and impaired insulin secretion or sensitivity leads insulin resistance (IR), respectively. Genetic and environmental factors attributed to the pathogenesis of DM and various therapeutic strategies are available for the prevention and treatment of T2DM.
“Diabetes mellitus (DM), a metabolic disorder is one of the most prevalent chronic diseases worldwide across developed as well as developing nations. Hyperglycemia is the core feature of the type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), following insulin deficiency and impaired insulin secretion or sensitivity leads insulin resistance (IR), respectively. Genetic and environmental factors attributed to the pathogenesis of DM and various therapeutic strategies are available for the prevention and treatment of T2DM.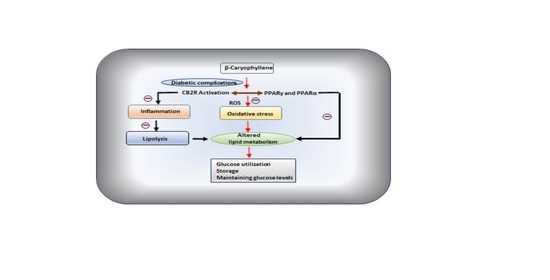
 “While activation of cannabinoid (CB2) receptors has been shown to be neuroprotective, no studies have examined whether this neuroprotection is directed at cerebral arterioles and no studies have examined whether activation of CB2 receptors can rescue cerebrovascular dysfunction during a chronic disease state such as type 1 diabetes (T1D).
“While activation of cannabinoid (CB2) receptors has been shown to be neuroprotective, no studies have examined whether this neuroprotection is directed at cerebral arterioles and no studies have examined whether activation of CB2 receptors can rescue cerebrovascular dysfunction during a chronic disease state such as type 1 diabetes (T1D). “Cannabidiol (CBD) is known for its vasorelaxant (including in the human pulmonary artery), anti-proliferative and anti-inflammatory properties. The aim of our study was to examine the potential preventive effect of chronic CBD administration (10 mg/kg/day for three weeks) on monocrotaline (MCT)-induced pulmonary hypertension (PH) rats.
“Cannabidiol (CBD) is known for its vasorelaxant (including in the human pulmonary artery), anti-proliferative and anti-inflammatory properties. The aim of our study was to examine the potential preventive effect of chronic CBD administration (10 mg/kg/day for three weeks) on monocrotaline (MCT)-induced pulmonary hypertension (PH) rats.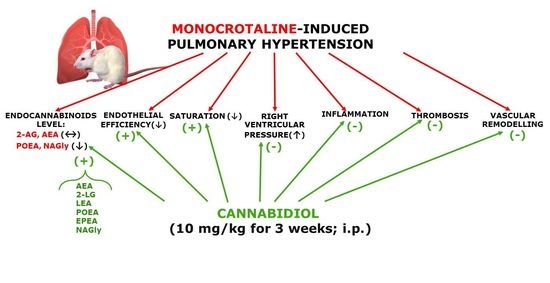
 “In recent years there has been a growing appreciation by regulatory authorities that cannabis-based medicines can play a useful role in disease therapy.
“In recent years there has been a growing appreciation by regulatory authorities that cannabis-based medicines can play a useful role in disease therapy. “Cannabidiol (CBD) is reported to produce pain relief, but the clinically relevant cellular and molecular mechanisms remain uncertain.
“Cannabidiol (CBD) is reported to produce pain relief, but the clinically relevant cellular and molecular mechanisms remain uncertain. “The prevalence of mild traumatic brain injury is highest amongst the adolescent population and can lead to complications including neuroinflammation and excitotoxicity.
“The prevalence of mild traumatic brain injury is highest amongst the adolescent population and can lead to complications including neuroinflammation and excitotoxicity.