“Fibromyalgia is a complex disease process that is as prevalent as it is poorly understood. Research into the pathophysiology is ongoing, and findings will likely assist in identifying new therapeutic options to augment those in existence today that are still insufficient for the care of a large population of patients.
“Fibromyalgia is a complex disease process that is as prevalent as it is poorly understood. Research into the pathophysiology is ongoing, and findings will likely assist in identifying new therapeutic options to augment those in existence today that are still insufficient for the care of a large population of patients.
Recent evidence describes the use of cannabinoids in the treatment of fibromyalgia.
This study provides a systematic, thorough review of the evidence alongside a review of the seminal data regarding the pathophysiology, diagnosis, and current treatment options.
Fibromyalgia is characterized by widespread chronic pain, fatigue, and depressive episodes without an organic diagnosis, which may be prevalent in up to 10% of the population and carries a significant cost in healthcare utilization, morbidity, a reduced quality of life, and productivity. It is frequently associated with psychiatric comorbidities. The diagnosis is clinical and usually prolonged, and diagnostic criteria continue to evolve. Some therapies have been previously described, including neuropathic medications, milnacipran, and antidepressants. Despite some level of efficacy, only physical exercise has strong evidence to support it.
Cannabis has been used historically to treat different pain conditions since ancient times.
Recent advances allowed for the isolation of the active substances in cannabis and the production of cannabinoid products that are nearly devoid of psychoactive influence and provide pain relief and alleviation of other symptoms. Many of these, as well as cannabis itself, are approved for use in chronic pain conditions.
Evidence supporting cannabis in chronic pain conditions is plentiful; however, in fibromyalgia, they are mostly limited. Only a handful of randomized trials exists, and their objectivity has been questioned. However, many retrospective trials and patient surveys suggest the significant alleviation of pain, improvement in sleep, and abatement of associated symptoms.
Evidence supporting the use of cannabis in chronic pain and specifically in fibromyalgia is being gathered as the use of cannabis increases with current global trends. While the current evidence is still limited, emerging data do suggest a positive effect of cannabis in fibromyalgia.
Cannabis use is not without risks, including psychiatric, cognitive, and developmental as well as the risks of addiction. As such, clinical judgment is warranted to weigh these risks and prescribe to patients who are more likely to benefit from this treatment. Further research is required to define appropriate patient selection and treatment regimens.”
https://pubmed.ncbi.nlm.nih.gov/33004171/
https://www.sciencedirect.com/science/article/pii/S1521689620300781?via%3Dihub

 “Fish oil (FO) and phytocannabinoids have received considerable attention for their intestinal anti-inflammatory effects.
“Fish oil (FO) and phytocannabinoids have received considerable attention for their intestinal anti-inflammatory effects. “Cannabidiol (CBD) is known for its vasorelaxant (including in the human pulmonary artery), anti-proliferative and anti-inflammatory properties. The aim of our study was to examine the potential preventive effect of chronic CBD administration (10 mg/kg/day for three weeks) on monocrotaline (MCT)-induced pulmonary hypertension (PH) rats.
“Cannabidiol (CBD) is known for its vasorelaxant (including in the human pulmonary artery), anti-proliferative and anti-inflammatory properties. The aim of our study was to examine the potential preventive effect of chronic CBD administration (10 mg/kg/day for three weeks) on monocrotaline (MCT)-induced pulmonary hypertension (PH) rats.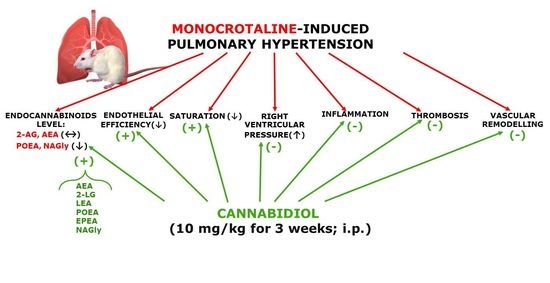
 “In recent years there has been a growing appreciation by regulatory authorities that cannabis-based medicines can play a useful role in disease therapy.
“In recent years there has been a growing appreciation by regulatory authorities that cannabis-based medicines can play a useful role in disease therapy. “Cannabis exposure is becoming more common in older age but little is known about how it is associated with brain health in this population.
“Cannabis exposure is becoming more common in older age but little is known about how it is associated with brain health in this population. “To determine if cannabis may be used as an alternative or adjunct treatment for intermittent and chronic prescription opioid users.
“To determine if cannabis may be used as an alternative or adjunct treatment for intermittent and chronic prescription opioid users. “The prevalence of mild traumatic brain injury is highest amongst the adolescent population and can lead to complications including neuroinflammation and excitotoxicity.
“The prevalence of mild traumatic brain injury is highest amongst the adolescent population and can lead to complications including neuroinflammation and excitotoxicity.