“Cannabidiol (CBD) and cannabigerol (CBG) are Cannabis sativa terpenophenols.
“Cannabidiol (CBD) and cannabigerol (CBG) are Cannabis sativa terpenophenols.
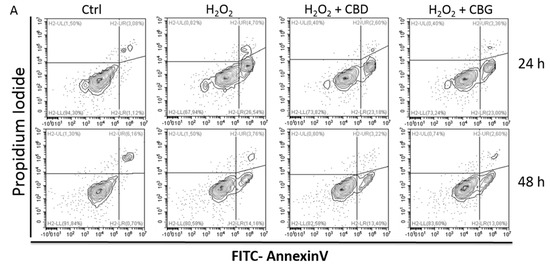
Although CBD’s effectiveness against neurological diseases has already been demonstrated, nothing is known about CBG. Therefore, a comparison of the effects of these compounds was performed in two experimental models mimicking the oxidative stress and neurotoxicity occurring in neurological diseases.
Rat astrocytes were exposed to hydrogen peroxide and cell viability, reactive oxygen species production and apoptosis occurrence were investigated. Cortexes were exposed to K+ 60 mM depolarizing stimulus and serotonin (5-HT) turnover, 3-hydroxykinurenine and kynurenic acid levels were measured. A proteomic analysis and bioinformatics and docking studies were performed.
Both compounds exerted antioxidant effects in astrocytes and restored the cortex level of 5-HT depleted by neurotoxic stimuli, whereas sole CBD restored the basal levels of 3-hydroxykinurenine and kynurenic acid. CBG was less effective than CBD in restoring the levels of proteins involved in neurotransmitter exocytosis. Docking analyses predicted the inhibitory effects of these compounds towards the neurokinin B receptor.
Conclusion: The results in the in vitro system suggest brain non-neuronal cells as a target in the treatment of oxidative conditions, whereas findings in the ex vivo system and docking analyses imply the potential roles of CBD and CBG as neuroprotective agents.”
https://pubmed.ncbi.nlm.nih.gov/32443623/
https://www.mdpi.com/1422-0067/21/10/3575

 “In this study cannabidiol (CBD) was administered orally to determine its effects and mechanisms in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS). We hypothesized that 75 mg/kg of oral CBD given for 5 days after initiation of disease would reduce EAE severity through suppression of either the early peripheral immune or late neuroimmune response.
“In this study cannabidiol (CBD) was administered orally to determine its effects and mechanisms in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS). We hypothesized that 75 mg/kg of oral CBD given for 5 days after initiation of disease would reduce EAE severity through suppression of either the early peripheral immune or late neuroimmune response.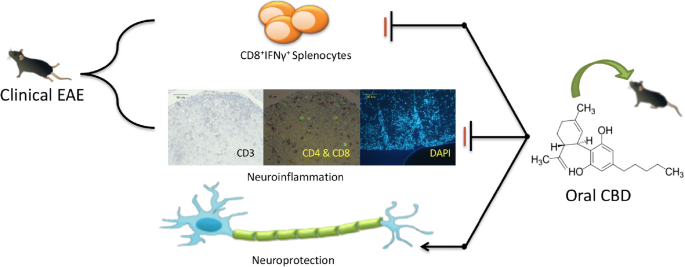
 “Cannabidiol (CBD), the major non-psychoactive constituent of Cannabis sativa L., has gained traction as a potential treatment for intractable chronic pain in many conditions. Clinical evidence suggests that CBD provides therapeutic benefit in certain forms of epilepsy and imparts analgesia in certain conditions, and improves quality of life.
“Cannabidiol (CBD), the major non-psychoactive constituent of Cannabis sativa L., has gained traction as a potential treatment for intractable chronic pain in many conditions. Clinical evidence suggests that CBD provides therapeutic benefit in certain forms of epilepsy and imparts analgesia in certain conditions, and improves quality of life.
 “Medical cannabis (MC) is becoming more and more popular among patients with chronic pain syndromes.
“Medical cannabis (MC) is becoming more and more popular among patients with chronic pain syndromes. “Two patient case reports are presented describing the use of cannabidiol (CBD) for the symptomatic relief of a lumbar compression fracture and in the mitigation of thoracic discomfort and dysesthesia secondary to a surgically resected meningioma.
“Two patient case reports are presented describing the use of cannabidiol (CBD) for the symptomatic relief of a lumbar compression fracture and in the mitigation of thoracic discomfort and dysesthesia secondary to a surgically resected meningioma. “The inhibitor of DNA binding (Id) proteins are regulators of cell cycle and cell differentiation. Of all Id family proteins, Id1 is mostly linked to tumorigenesis, cellular senescence as well as cell proliferation and survival.
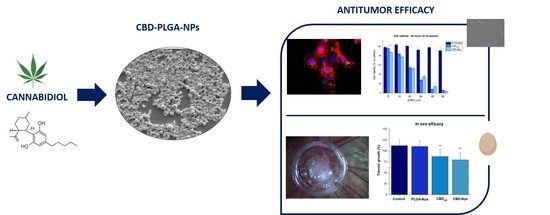
“The inhibitor of DNA binding (Id) proteins are regulators of cell cycle and cell differentiation. Of all Id family proteins, Id1 is mostly linked to tumorigenesis, cellular senescence as well as cell proliferation and survival. “The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.
“The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.